The 13Th SEGJ International Symposium / Technical Progam
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

2010 FIFA World Cup South Africa™ Teams
2010 FIFA World Cup South Africa™ Teams Statistical Kit 1 (To be used in conjunction with Match Kit) Last update: 5 June 2010 Next update: 10 June 2010 Contents Participants 2010 FIFA World Cup South Africa™..........................................................................................3 Global statistical overview: 32 teams at a glance..........................................................................................4 Algeria (ALG) ...................................................................................................................................................4 Argentina (ARG) ..............................................................................................................................................8 Australia (AUS)...............................................................................................................................................12 Brazil (BRA) ....................................................................................................................................................16 Cameroon (CMR)...........................................................................................................................................20 Chile (CHI) .....................................................................................................................................................23 Côte d’Ivoire (CIV)..........................................................................................................................................26 -
Workshop Program
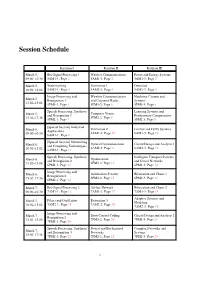
Session Schedule Kaiulani I Kaiulani II Kaiulani III March 5, Bio-Signal Processing 1 Wireless Communications Power and Energy Systems 09:00–10:30 5AM1-1: Page 2 5AM1-2: Page 2 5AM1-3: Page 3 March 5, Watermarking Estimation 1 Detection 10:50–12:02 5AM2-1: Page 4 5AM2-2: Page 4 5AM2-3: Page 5 Image Processing and Wireless Communications Nonlinear Circuits and March 5, Recognition 1 and Cognitive Radio Systems 13:20–15:08 5PM1-1: Page 5 5PM1-2: Page 6 5PM1-3: Page 6 Speech Processing, Synthesis Learning Systems and Computer Vision March 5, and Recognition 1 Evolutionary Computations 5PM2-2: Page 8 15:30–17:36 5PM2-1: Page 7 5PM2-3: Page 8 [Special Session] Industrial March 6, Estimation 2 Control and Fuzzy Systems Applications 09:00–10:30 6AM1-2: Page 10 6AM1-3: Page 10 6AM1-1: Page 9 [Special Session] Networking Optical Communications Circuit Design and Analysis 1 March 6, and Computing Technologies 6AM2-2: Page 11 6AM2-3: Page 12 10:50–12:02 6AM2-1: Page 11 Speech Processing, Synthesis Intelligent Transport Systems Optimization March 6, and Recognition 2 and Sensor Networks 6PM1-2: Page 13 13:20–15:08 6PM1-1: Page 12 6PM1-3: Page 14 Image Processing and Information Security Bifurcation and Chaos 1 March 6, Recognition 2 6PM2-2: Page 15 6PM2-3: Page 16 15:30–17:36 6PM2-1: Page 14 March 7, Bio-Signal Processing 2 Ad-hoc Network Bifurcation and Chaos 2 09:00–10:30 7AM1-1: Page 16 7AM1-2: Page 17 7AM1-3: Page 18 Adaptive Systems and Filters and Oscillation Estimation 3 March 7, Modeling 7AM2-1: Page 18 7AM2-2: Page 19 10:50–12:02 7AM2-3: Page 19 Image Processing and Error Control Coding Circuit Design and Analysis 2 March 7, Recognition 3 7PM1-2: Page 20 7PM1-3: Page 21 13:20–15:08 7PM1-1: Page 20 Speech Processing, Synthesis Neural and Bio-Inspired Complex Networks and March 7, and Recognition 3 Networks Systems 15:30–17:18 7PM2-1: Page 22 7PM2-2: Page 22 7PM2-3: Page 23 1 5AM1-1: Bio-Signal Processing 1 Date: March 5, 9:00–10:30 Room: Kaiulani I Chair: Prof. -

Furthering National Development Through Sport, the Case of Qatar Wadih Ishac
Furthering national development through sport, the case of Qatar Wadih Ishac To cite this version: Wadih Ishac. Furthering national development through sport, the case of Qatar. Business administra- tion. Université Bourgogne Franche-Comté, 2018. English. NNT : 2018UBFCH037. tel-02071444 HAL Id: tel-02071444 https://tel.archives-ouvertes.fr/tel-02071444 Submitted on 18 Mar 2019 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. UNIVERSITE DE BOURGOGNE UFR des Sciences et Techniques des Activités Physiques et Sportives THÈSE Pour obtenir le grade de Docteur de l’Université de Bourgogne Discipline : Sciences et Techniques des Activités Physiques et Sportives Par ISHAC Wadih August 2018 Furthering national development through sport, the case of Qatar Directeur de thèse : BOUCHET Patrick, Professeur des Universités, Université de Bourgogne Franche-Comté Co-directeur de thèse : SOBRY Claude, Professeur des Universités, Université de Lille 2 Jury : Président : Mohammed Kaach, Professeur, Institut National des Sports Moulay Rachid Rapporteurs : Ati Abdessatar Professeur, Jazan University Kaouther Kooli Professeur, Bournemouth University Sorina Cernaianu,Professeur, University of Craiova Win if you can Lose if you must But never QUIT Cameron Trammell Declaration This dissertation is the result of my own work and includes nothing which is the outcome of work done in collaboration, except where specifically indicated in the text. -

Editorial Original Articles Review Article Case Report Clinical Topics
Vol.49, No.11&12 November/December 2006 Editorial New Surgical Robotics for Clinical Use in Neurosurgery Makoto Hashizume................................................................................................................................................ 333 Original Articles Development of Surgical Manipulator System “HUMAN” for Clinical Neurosurgery Koji Nishizawa, Masakatsu G Fujie, Kazuhiro Hongo, Takeyoshi Dohi, Hiroshi Iseki ............................. 335 An Analysis of Ambulance-transported Cases of Attempted Suicide in 3 Prefectures (Akita, Aomori, and Iwate) in the Northern Tohoku Area in Japan Chikara Yonekawa, Hajime Nakae, Kimitaka Tajimi, Yutaka Motohashi, Yasushi Asari, Shigeatsu Endo, Sergio A Perez Barrero ........................................................................................................... 345 Gender Differences in Symptom Experience at End-of-Life among Elderly Patients Dying at Home with Advanced Cancer in Japan Yoshihisa Hirakawa, Yuichiro Masuda, Masafumi Kuzuya, Akihisa Iguchi, Kazumasa Uemura ................................................................................................................................................ 351 Change of Plasma High Sensitive —C reactive protein levels in climbers Shintaro Suzuki, Yuji Kiuchi, Tetsuya Nemoto, Kenta Kobayashi, Hidekazu Ota ..................................... 358 The Effectiveness of a New Law to Reduce Alcohol-impaired Driving in Japan Takashi Nagata, David Hemenway, Melissa J Perry ....................................................................................... -

Statistical Kit
FIFA Confederations Cup Statistical Kit STATS Kit Content Introduction.......................................................................................................................................................3 Continental showdown..........................................................................................................................3 Winners at a glance ...............................................................................................................................3 Finals .....................................................................................................................................................3 The History ........................................................................................................................................................4 Competition Superlatives.................................................................................................................................6 Most participations ................................................................................................................................6 Most wins..............................................................................................................................................6 Most matches ........................................................................................................................................6 Most goals.............................................................................................................................................6 -

PACIFIC RIM CUP LAUNCHES in HONOLULU in 2018 World-Class Soccer Leagues to Clash at Aloha Stadium on Feb
Takehiko Nakamura FOR IMMEDIATE RELEASE President/CEO, Blue United Corp. November 13, 2017 [email protected] PACIFIC RIM CUP LAUNCHES IN HONOLULU IN 2018 World-class soccer leagues to clash at Aloha Stadium on Feb. 8 and 10. HONOLULU – International soccer stars from Japan, Canada and the USA are geared up and ready to clash in the 1st annual Pacific Rim Cup, held Feb. 8 and 10, 2018, at Aloha Stadium in Hawaii. This marks the first time in six years that the Japan Professional Football League (J.League) and Major League Soccer (MLS) will compete in Hawai'i. DOME Corporation, the exclusive partner of Under Armour in Japan, is the Title Sponsor of the Pacific Rim Cup. Blue United Corporation is the host organization for the inaugural Pacific Rim Cup. Blue United President, Takehiko Nakamura states, “We are pleased to announce the Pacific Rim Cup. Our hope is that this tournament will be an annual event that the local community can be proud of, and look forward to, with the vision to expand within the Pacific Rim region and showcase Hawaii as the destination to host international sporting events.” The four teams competing include the Vancouver Whitecaps FC and Columbus Crew SC from MLS and Hokkaido Consadole Sapporo and Iwaki FC from the J.League. In May 2017, Hokkaido Prefecture and the State of Hawaii signed a sister-state relationship. Iwaki City is a sister city with the island of Kauai. Tournament Schedule / Kickoff Times: Thursday, February 8, 2018, at Aloha Stadium: 1st Game: 5 p.m. – Vancouver vs. -

O UWAGA KONKURS Po Raz Ósmy PREMIER W KALISZU Kultura
cg UWAGA <5 N JarocińskaiNr 19 (397), 8 maja 1998 KONKURS CD ISSN 1230-851X TYGODNIK ZIEMI JAROCIŃSKIEJ nr indeksu 343 82X 4 0 0 zł na 4 0 0 numer Cena 1,50 zł____________ O Szczegóły w następnym numerze Rytm i Melodia PREMIER W KALISZU Po raz ósmy Premier Jerzy Buzek, wicepremier Janusz Tomaszewski i przewodniczący N S Z Z “Solidarność” Marian Krzaklewski wzięli udział 1 maja w V I Ogólnopolskiej Pielgrzymce Ludzi Pracy do bazyliki Osiemdziesięciu wykonawców z ośmiu krajów weźmie św. Józefa w Kaliszu. W uroczystościach uczestniczyło ponad 3 tys. osób. Wśród nich byli udział w V III Międzynarodowym Przeglądzie Piosenki przedstawiciele górniczej i hutniczej “Solidarności”, stoczniowcy, marynarze, a także Dziecięcej “ Rytm i Melodia” . Od 8 do 10 maja rywalizować kilkunastoosobowa delegacja z Jarocina. będą o Grand Prix burmistrza Jarocina. Dokończenie na str. 5 Młodzi artyści z Rosji, Armenii, Węgier, Francji, Czech, Bułgarii, Słowacji i Polski wystąpią na kolejnym festiwalu “Rytm i Melodia”. Przeglądy konkursowe odbędą się w JOK-u. W piątek o 17.00 artyści zaprezentują się w programie “Dyliżans międzynarodowy”, a w sobotę o tej samej godzinie odjedzie “Dyliżans Polski”. W niedzielę o 19.00 w amfiteatrze wszyscy spotkają się w “Rozśpiewanym dyliżansie”. Wykonawcom towarzyszyć będzie tradycyjnie grupa “Harry Band”. Bilety na koncerty w JOK-u kosztują 4 zł. W razie niepogody koncert z amfiteatru będzie przeniesiony do JOK-u. Z kościoła do pożaru P on ad d w a h e k ta ry m io d n ik a sosnow ego s p a liło się 2 m a ja w Leśnictwie Cielcza. -

National Identity and Global Sports Events SUNY Series on Sport, Culture, and Social Relations CL Cole and Michael A
edited by alan tomlinson and christopher young National Identity and Global Sports Events SUNY series on Sport, Culture, and Social Relations CL Cole and Michael A. Messner, editors National Identity and Global Sports Events Culture, Politics, and Spectacle in the Olympics and the Football World Cup Edited by Alan Tomlinson and Christopher Young State University of New York Press Published by State University of New York Press, Albany © 2006 State University of New York All rights reserved Printed in the United States of America No part of this book may be used or reproduced in any manner whatsoever Without written permission. No part of this book may be stored in a retrieval system or transmitted in any form or by any means including electronic, electrostatic, magnetic tape, mechanical, photocopying, recording, or otherwise without the prior permission in writing of the publisher. For information, address State University of New York Press, 194 Washington Avenue, Suite 305, Albany, NY 12210-2384 Production by Michael Haggett Marketing by Michael Campochiaro Library of Congress Cataloging-in-Publication Data National identity and global sports events / culture, politics, and spectacle in the Olympics and the football World Cup / edited by Alan Tomlinson and Christopher Young. p. cm. — (SUNY series on sport, culture, and social relations) Includes bibliographical references and index. ISBN 0-7914-6615-9 (hardcover : alk. paper) 1. Nationalism and sports—History. 2. Sports and globalization—History. 3 Sports—Sociological aspects—Cross-cultural studies. I. Tomlinson, Alan. II. Young, Christopher, 1967– III. Series. GV706.34.N38 2005 306.4'83—dc22 2004029962 ISBN-13: 978-0-7914-6615-5 (hardcover : alk. -

Yamaha Motor Monthly Newsletter
Yamaha Motor Monthly Newsletter YAMAHA ASEAN CUP U‐13 FOOTBALL (2013) Spotlight: Sports Activities April 15, 2014 (Issue No. 16) Sports Activities Working in the communities to share Kando worldwide In its golden years, the Yamaha Football Club “Jubilo Iwata” won both halves of the 2002 season of the “J. League,” Japan’s professional football league, to become the uncontested league champions © Jubilo Yamaha Motor is a company dedicated to the mission of bringing Kando* and a more fulfilling life to people all over the world through the products we build. To accomplish this, in addition to the wide variety of products we offer that ranges from motorcycles, snowmobiles, ATVs and racing karts to motorboats, sailboats and personal watercraft, we have also actively promoted motorsport competitions, recreational and sporting events and product training programs. Through these activities, we have progressively built Yamaha’s sporty brand image. At the same time, we have spread our involvement beyond the realms of our products and business areas to include programs in major sports like football and rugby and other popular sports where we can pioneer “new Kando.” These efforts help in bringing together and motivating the employees of our group companies and affiliated business partners, as well as building bonds with the communities we live and work in and promoting and strengthening the Yamaha brand. In this issue, we introduce some of the sports activities that help spread and enrich the Yamaha brand image. *Kando is a Japanese word for the simultaneous feelings of deep satisfaction and intense excitement that we experience when we encounter something of exceptional value. -

Furthering National Development Through Sport, the Case of Qatar Wadih Ishac
Furthering national development through sport, the case of Qatar Wadih Ishac To cite this version: Wadih Ishac. Furthering national development through sport, the case of Qatar. Business administra- tion. Université Bourgogne Franche-Comté, 2018. English. NNT : 2018UBFCH037. tel-02071444 HAL Id: tel-02071444 https://tel.archives-ouvertes.fr/tel-02071444 Submitted on 18 Mar 2019 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. UNIVERSITE DE BOURGOGNE UFR des Sciences et Techniques des Activités Physiques et Sportives THÈSE Pour obtenir le grade de Docteur de l’Université de Bourgogne Discipline : Sciences et Techniques des Activités Physiques et Sportives Par ISHAC Wadih August 2018 Furthering national development through sport, the case of Qatar Directeur de thèse : BOUCHET Patrick, Professeur des Universités, Université de Bourgogne Franche-Comté Co-directeur de thèse : SOBRY Claude, Professeur des Universités, Université de Lille 2 Jury : Président : Mohammed Kaach, Professeur, Institut National des Sports Moulay Rachid Rapporteurs : Ati Abdessatar Professeur, Jazan University Kaouther Kooli Professeur, Bournemouth University Sorina Cernaianu,Professeur, University of Craiova Win if you can Lose if you must But never QUIT Cameron Trammell Declaration This dissertation is the result of my own work and includes nothing which is the outcome of work done in collaboration, except where specifically indicated in the text. -

To Detect Neuroendocrine Prostate Cancer Genomic and DNA Methylation Changes
PROSTATE CANCER - ADVANCED 8 General Session, Thu, 3:00 PM-4:25 PM Circulating tumor DNA (ctDNA) to detect neuroendocrine prostate cancer genomic and DNA methylation changes. Himisha Beltran, Alessandro Romanel, Vincenza Conteduca, Nicola Casiraghi, Michael Sigouros, Gian Marco Franceschini, Tarcisio Fedrizzi, sheng-Yu Ku, Alicia Alonso, Juan Miguel Mosquera, Emma Dann, Andrea Sboner, Jenny Xiang, Olivier Elemento, David M. Nanus, Scott T. Tagawa, Matteo Benelli, Francesca Demichelis; Dana-Farber Cancer Institute, Boston, MA; University of Trento, Trento, Italy; Dana Farber Cancer Institute, Boston, MA; Department of Medicine, Division of Medical Oncology, Weill Cornell Medicine, New York City, NY; Weill Cornell Medicine, New York, NY; Department of Pathology & Laboratory Medicine, Englander Institute for Precision Medicine, Weill Cornell Medical College & New York-Presbyterian Hospital, New York, NY; Weill Cornell Medical College, New York, NY; Department of Physiology and Biophysics, Institute for Computational Biomedicine, Englander Institute for Precision Medicine, Weill Cornell Medical College, New York, NY; Sandra and Edward Meyer Cancer Center, New York, NY; Department of Cellular, Computational and Integrative Biology, University of Trento, Trento, Italy Background: Loss of androgen receptor signaling dependence occurs in approximately 20% of treatment resistant prostate cancers, and this may manifest as transformation to castration resistant neuroendocrine prostate cancer (CRPC-NE). The diagnosis of CRPC-NE relies on a metastatic tumor biopsy, which is invasive for patients. Here we study ctDNA as an approach to identify CRPC-NE-associated genomic and epigenomic changes. We also use this as a tool to understand tumor heterogeneity and clonal dynamics that occurs during CRPC-NE progression. Methods: 64 patients with metastatic prostate cancer (10 hormone naive, 35 castration resistant adenocarcinoma (CRPC-Adeno), 17 CRPC-NE) were prospectively enrolled for matched metastatic biopsy and blood collection. -

5 Heures Pour Juger Djemaï L Moins De Cinq Heures De Temps Ont Suffi Pour Juger L’Ancien Secrétaire Général Du FLN, Mohamed Djemaï, Pour Une Affaire Très
l TRIBUNAL DE SIDI-M’HAMED 5 heures pour juger Djemaï l Moins de cinq heures de temps ont suffi pour juger l’ancien secrétaire général du FLN, Mohamed Djemaï, pour une affaire très différente de celles des autres personnalités arrêtées qui défilent depuis de longs mois devant le tribunal de Sidi-M’hamed. PAGE 4 l BAISSE CONTINUE DES CAS DE CONTAMINATION Covid-19 : faut-il Edition du Centre - ISSN IIII 0074 Edition du Centre ICI, ALGER Par Naoufel Brahimi El Mili (P. 6) crierl La tendance baissière des cas devictoire Covid-19, même si elle se confirme, ne pousse pas les acteurs ? du secteur de la santé à un optimisme démesuré. La bataille, disent-ils, est loin d’être encore gagnée. Et pour cause, de grands défis attendent encore l’Algérie : la rentrée scolaire, la reprise du transport entre les wilayas mais également la réouverture des frontières constitueront de véritables tests. PAGE 5 BILLET (PAS) DOUX Qu'avez-vous commis que nous ignorons, mon Général ! Je n'ai pas vu le reportage de M6 sur l'Algérie. Je tenais à suivre l'interview du Président Tebboune qui passait à la même heure. Mon ami Nacer Belhadjoudja, l'un des deux intervie- weurs, m'avait appelé la veille pour m'an- noncer qu'il poserait une question sur Khaled Drareni, ce jeune Algérien qui, de notre point de vue, ne mérite pas deux années de prison. À moins qu'on nous cache des choses ! J'aurais aimé que l'on évoque aussi l'em- prisonnement de Nekkaz ou de Ali Ghediri.