To Detect Neuroendocrine Prostate Cancer Genomic and DNA Methylation Changes
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

ARUP Laboratories & the University of Utah Department Of
& ARUP LABORATORIES & THE UNIVERSITY OF UTAH DEparTMENT OF paTHOLOGY March 2014 Message from the President & CEO ARUP Leadership About ARUP what’s Culture of Innovation inside Automation Initiative Unique Relationships ARUP Medical Directors & Consultants ARUP History & Timeline CONSISTENCY, SUCCESSS, EXPERIENCE ARUP LEADERSHIP ARUP BOarD OF DIRECTORS ARUP EXECUTIVE TEAM Peter E. JENSEN, MD Peter E. JENSEN, MD Chairman Chair, Department of Pathology, University of Utah Chair, Department of Pathology, University of Utah EDWard R. ASHWood, MD EDWard R. ASHWood, MD President and CEO President and CEO Julie AltWieS, BS H. ROGer BOYer Vice President, Director of Sales The Boyer Company NaNCY ANdeS, MBA, MT(ASCP) ARNold B. Combe Senior Vice President, Marketing University of Utah LESlie T. HamiltoN, MT(ASCP)SM CLARK D. IVORY Senior Vice President, Technical Operations University of Utah JerrY W. HUSSONG, MD, MS SuSAN D’ANjou JohNSON Vice President, Chief Medical Officer/Director of Futura Industries Laboratories Carl R. KjeldSberG, MD BriaN R. JackSON, MD, MS University of Utah Vice President, Chief Medical Informatics Officer LeeANNE B. LINdermaN David P. JackSON, MBA Zions Bank Senior Vice President, Strategic Services JameS L. MacFarlaNE JohN R. PENroSE, BS IC Group Senior Vice President, Chief Information Officer JohN K. MorriS Sherrie L. PerkiNS, MD, PHD Non-voting Member Senior Vice President, Research and Development, Office of General Counsel, University of Utah Co-Executive Director, ARUP Institute for Clinical and Experimental Pathology ANdreW A. Theurer, CPA Board Secretary KhoSroW ShotorbaNI, MBA, MT(ASCP) Senior Vice President, Business Innovations SHERRIE L. PERKINS, MD, PHD Invited Guest ANdreW A. Theurer, CPA, BA Senior Vice President, Chief Financial Officer CONSISTENCY, SUccESS, experience EXECUTIVE MANAGEMENT TEAM The executive management team’s maturity and devotion to patient care and leadership, from both the medical and business sides of health care, sustains ARUP as a valuable asset to its clients and the lab industry. -

The University of Utah a Component Unit of the State of Utah 2017
ANNUAL FINANCIAL REPORT THE UNIVERSITY OF UTAH A COMPONENT UNIT OF THE STATE OF UTAH 2017 TABLE OF CONTENTS Message from the President 2 - 3 Independent State Auditor’s Report 4 - 5 Management’s Discussion and Analysis 6 - 14 Financial Statements 15 - 20 Statement of Net Position 16 - 17 Statement of Revenues, Expenses, and Changes in Net Position 18 Statement of Cash Flows 19 - 20 Notes to Financial Statements 21 - 46 Required Supplementary Information 47 - 49 Governing Boards and Officers 50 Message from the President David W. Pershing ne hundred years ago, John A. Widtsoe, president of the University of Utah, declared that he hoped to see “this institution enter into the very life of our state; to help solve its problems, to Opoint its way, to help bear its burdens as well as to share in its prosperity…” It was understood then, as it is today, the responsibility of the flagship institution extends beyond its classrooms and laboratories. The University of Utah has become a world-class research and teaching institution, an engine of economic prosperity, and a provider of nationally recognized medical care. The U plays an integral role within the state, as was hoped, but the university’s positive influence now has a global impact. The success of the University of Utah is reliant on the responsible stewardship of intellectual, physical, and financial resources. We gratefully acknowledge critical contributions made by the residents and elected leaders of this state, as well as the Utah State Board of Regents and our Board of Trustees. The U excels because of their support. -

Androgen Receptor Targeted Agents for Castration Resistant Prostate Cancer: a Review of Clinical Effectiveness and Cost- Effectiveness
CADTH RAPID RESPONSE REPORT: SUMMARY WITH CRITICAL APPRAISAL Androgen Receptor Targeted Agents for Castration Resistant Prostate Cancer: A Review of Clinical Effectiveness and Cost- Effectiveness Service Line: Rapid Response Service Version: 1.0 Publication Date: June 6, 2019 Report Length: 30 Pages Authors: Khai Tran, Suzanne McCormack Cite As: Androgen Receptor Targeted Agents for Castration Resistant Prostate Cancer: A Review of Clinical Effectiveness and Cost-Effectiveness. Ottawa: CADTH; 2019 Jun. (CADTH rapid response report: summary with critical appraisal). ISSN: 1922-8147 (online) Disclaimer: The information in this document is intended to help Canadian health care decision-makers, health care professionals, health systems leaders, and policy-makers make well-informed decisions and thereby improve the quality of health care services. While patients and others may access this document, the document is made available for informational purposes only and no representations or warranties are made with respect to its fitness for any particular purpose. The information in this document should not be used as a substitute for professional medical advice or as a substitute for the application of clinical judgment in respect of the care of a particular patient or other professional judgment in any decision-making process. The Canadian Agency for Drugs and Technologies in Health (CADTH) does not endorse any information, drugs, therapies, treatments, products, processes, or services. While care has been taken to ensure that the information prepared by CADTH in this document is accurate, complete, and up-to-date as at the applicable date the material was first published by CADTH, CADTH does not make any guarantees to that effect. -

Medical & Technical
MEDICAL & TECHNICAL ARUP Expertise | 2019 JULY 2019 aruplab.com Information in this brochure is current as of July 2019. All content is subject to change. Please contact ARUP Client Services at (800) 522-2787 with any questions or concerns. ARUP supports our clients’ success by providing excellence and consistency in our delivery of services, by sharing knowledge, and by developing progressive laboratory technology. ARUP LABORATORIES ARUP believes in collaborating, sharing knowledge, and contributing to laboratory science in ways that provide the best value for the patient. We operate 24 hours per day, every day of the year, and are a one-stop shop. More than 99 percent of our test menu is performed in-house, providing greater efficiency and standardized test results. ARUP offers one of the broadest test menus in the industry, encompassing more than 3,000 tests and test combinations, including highly specialized and esoteric assays. ARUP’s Laboratory Test Directory contains complete, up-to-date test information, including methodology and reporting times, reference intervals, test notes, and CPT codes. More than 100 medical consultants and experts—nationally and internationally recognized pathologists, subspecialty-qualified clinicians, and board-certified clinical scientists—are available for client consultation. These professionals hold faculty appointments at the University of Utah School of Medicine and make significant contributions in research and development. Many participate in care teams at the Huntsman Cancer Hospital and Primary Children’s Hospital. We are one of the most automated laboratories in the United States, and much of our automation is unique, existing nowhere else in the world. ARUP’s automation would not perform with the intended quality if it were not for the carefully designed and engineered software that integrates separate components into one seamless system. -

Facilities and Other Resources - Overall
FACILITIES AND OTHER RESOURCES - OVERALL The necessary infrastructure at the University of Utah (prime performance site) and other participating sites (Table 1) are available to support the application, Utah Center for Clinical and Translational Science (Utah CCTS). The assembled team for this innovative center is comprised of experienced principal investigators, mentors and other stakeholders committed to supporting the vision and mission of the Clinical and Translational Science Award (CTSA) consortium. The Principal Investigators, Co-Investigators, and other personnel have the necessary organizational and administrative infrastructure to successfully develop, implement, and evaluate center programs to support the national CTSA consortium. Table 1. Utah CCTS Facilities and Other The Genetic Science Learning Center 4.A Resources Office for Equity and Diversity 4.B Resource Section Center for Law and Biomedical Sciences 4.C Intermountain West 1 The Center for Medical Innovation 4.D The Intermountain West 1.A Vice President’s Clinical & Translational 4.E State of Utah 1.B Scholars Program Salt Lake City 1.C University of Utah Molecular Medicine 4.F University of Utah (U of Utah) and 2 Program University of Utah Health (U Health) Department of Population Health 4.G University Hospital 2.A Sciences Community Clinics 2.B Department of Biomedical Informatics 4.H Huntsman Cancer Institute (Laboratory) 2.C • Biomedical Natural Language • Cancer Biostatistics 2.C.1 Processing 4.H.1 • Pedigree and Population Resource 2.C.2 Entertainment Arts and Engineering 4.I Huntsman Cancer Hospital 2.D Program and GApp Lab University Orthopaedic Center 2.E U Health Core Facilities 5 University Neuropsychiatric Institute 2.F Administration 5.A John A. -

2010 FIFA World Cup South Africa™ Teams
2010 FIFA World Cup South Africa™ Teams Statistical Kit 1 (To be used in conjunction with Match Kit) Last update: 5 June 2010 Next update: 10 June 2010 Contents Participants 2010 FIFA World Cup South Africa™..........................................................................................3 Global statistical overview: 32 teams at a glance..........................................................................................4 Algeria (ALG) ...................................................................................................................................................4 Argentina (ARG) ..............................................................................................................................................8 Australia (AUS)...............................................................................................................................................12 Brazil (BRA) ....................................................................................................................................................16 Cameroon (CMR)...........................................................................................................................................20 Chile (CHI) .....................................................................................................................................................23 Côte d’Ivoire (CIV)..........................................................................................................................................26 -

BULLETIN April 24, 2007 SERVING TOOELE COUNTY SINCE 1894 VOL
FRONT PAGE A1 www.tooeletranscript.com TUESDAY Family fights fires through the generations See B1 TOOELETRANSCRIPT BULLETIN April 24, 2007 SERVING TOOELE COUNTY SINCE 1894 VOL. 113 NO. 96 50¢ Hospital to expand Addition to Mountain West Medical Center will cater to women’s health needs by Mark Watson women can meet to learn the lat- STAFF WRITER est in the world of medicine and A new $4.5 million addition to treatment. Mountain West Medical Center Of the total cost of the new will double the facility’s ability to addition, $1.4 million will be spent care for female patients, accord- on state-of-the-art medical equip- ing to hospital CEO Chuck Davis. ment. Hospital officials announced Davis said market demand was the expansion plans at the behind the decision to expand. Healthy Woman Wellness Fair “The hospital opened on May Thursday night at Tooele High 17, 2002, and during the first year School. The new building will be we had 225 deliveries,” Davis said. located near the main hospital on “During 2006, we had 480 deliv- the northwest side and will have eries. Something needed to be a separate entrance and waiting done.” room, according to Davis. The Davis said the women of the 14,000-square-foot facility should world drive the medical services be completed by the fall of 2008. industry. The hospital is working on archi- “About 70 to 80 percent of med- tectural designs and will seek con- ical decisions are made by women struction bids later in the year. in the community,” he said. -

February 13–15, 2020 Moscone West Building | San Francisco, California
gucasym.org #GU20 IN PURSUIT OF PATIENT-CENTERED CARE PROCEEDINGS FEBRUARY 13–15, 2020 MOSCONE WEST BUILDING | SAN FRANCISCO, CALIFORNIA COSPONSORED BY 2020 Genitourinary Cancers Symposium Proceedings Guide to Abstracts Penile Cancer 1 Prostate Cancer—Advanced 3 Prostate Cancer—Localized 69 Testicular Cancer 96 Urethral Cancer 106 Urothelial Carcinoma 109 Renal Cell Cancer 151 The abstracts contained within are published as an online supplement to Journal of Clinical Oncology (JCO) and carry JCO citations. The format for citation of abstracts is as follows: J Clin Oncol 38, 2020 (suppl 6; abstr 1). Editor: Michael A. Carducci, MD The 2020 Genitourinary Cancers Symposium Proceedings (ISBN 978-0-9996799-9-9) is published by the American Society of Clinical Oncology. Requests for permission to reprint abstracts should be directed to [email protected]. Editorial correspondence should be directed to [email protected]. Copyright © 2020 American Society of Clinical Oncology. All rights reserved. No part of this publication may be reproduced or transmitted in any form orby any means, electronic or mechanical, including photocopy, recording, or any information storage and retrieval system, without written permission by the Society. The abstracts contained within are copyrighted to Journal of Clinical Oncology and are reprinted within the Proceedings with Permissions. The American Society of Clinical Oncology assumes no responsibility for errors or omissions in this publication. The reader is advised to check the appropriate medical literature and the product information currently provided by the manufacturer of each drug to be administered to verify the dosage, the method and duration or administration, or contraindications. It is the responsibility of the treating physician or other health care professional, relying on independent experience and knowledge of the patient, to determine drug, disease, and the best treatment for the patient. -
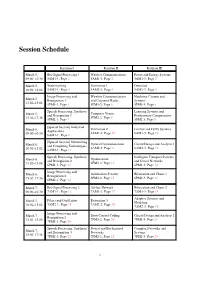
Workshop Program
Session Schedule Kaiulani I Kaiulani II Kaiulani III March 5, Bio-Signal Processing 1 Wireless Communications Power and Energy Systems 09:00–10:30 5AM1-1: Page 2 5AM1-2: Page 2 5AM1-3: Page 3 March 5, Watermarking Estimation 1 Detection 10:50–12:02 5AM2-1: Page 4 5AM2-2: Page 4 5AM2-3: Page 5 Image Processing and Wireless Communications Nonlinear Circuits and March 5, Recognition 1 and Cognitive Radio Systems 13:20–15:08 5PM1-1: Page 5 5PM1-2: Page 6 5PM1-3: Page 6 Speech Processing, Synthesis Learning Systems and Computer Vision March 5, and Recognition 1 Evolutionary Computations 5PM2-2: Page 8 15:30–17:36 5PM2-1: Page 7 5PM2-3: Page 8 [Special Session] Industrial March 6, Estimation 2 Control and Fuzzy Systems Applications 09:00–10:30 6AM1-2: Page 10 6AM1-3: Page 10 6AM1-1: Page 9 [Special Session] Networking Optical Communications Circuit Design and Analysis 1 March 6, and Computing Technologies 6AM2-2: Page 11 6AM2-3: Page 12 10:50–12:02 6AM2-1: Page 11 Speech Processing, Synthesis Intelligent Transport Systems Optimization March 6, and Recognition 2 and Sensor Networks 6PM1-2: Page 13 13:20–15:08 6PM1-1: Page 12 6PM1-3: Page 14 Image Processing and Information Security Bifurcation and Chaos 1 March 6, Recognition 2 6PM2-2: Page 15 6PM2-3: Page 16 15:30–17:36 6PM2-1: Page 14 March 7, Bio-Signal Processing 2 Ad-hoc Network Bifurcation and Chaos 2 09:00–10:30 7AM1-1: Page 16 7AM1-2: Page 17 7AM1-3: Page 18 Adaptive Systems and Filters and Oscillation Estimation 3 March 7, Modeling 7AM2-1: Page 18 7AM2-2: Page 19 10:50–12:02 7AM2-3: Page 19 Image Processing and Error Control Coding Circuit Design and Analysis 2 March 7, Recognition 3 7PM1-2: Page 20 7PM1-3: Page 21 13:20–15:08 7PM1-1: Page 20 Speech Processing, Synthesis Neural and Bio-Inspired Complex Networks and March 7, and Recognition 3 Networks Systems 15:30–17:18 7PM2-1: Page 22 7PM2-2: Page 22 7PM2-3: Page 23 1 5AM1-1: Bio-Signal Processing 1 Date: March 5, 9:00–10:30 Room: Kaiulani I Chair: Prof. -

Genomic Pro Ling in Ctdna Revealing Complicated Resistance
Genomic Proling in ctDNA Revealing Complicated Resistance Mechanism of Olaparib and Abiraterone as Salvage Therapy in Prostate Cancer: A Case Report Fang Yuan Chongqing University Nan Liu Chongqing University Hong Luo Chongqing University XiaoTian Zhang BGI MingZhen Yang Army Medical University Hong Zhou ( [email protected] ) Chongqing University https://orcid.org/0000-0003-2526-8153 Case Report Keywords: mCRPC, Olaparib, PALB2 Posted Date: July 9th, 2020 DOI: https://doi.org/10.21203/rs.3.rs-38627/v1 License: This work is licensed under a Creative Commons Attribution 4.0 International License. Read Full License Page 1/12 Abstract Background: PARP inhibitor, e.g. Olaparib, displayed superior clinical effect in metastatic castration prostate cancer (mCRPC) patients with deleterious mutations of DNA damage repair genes (DDR) as reported recently. Besides, for mCRPC patients without DDR alterations, a combination of Olaparib and abiraterone may achieve an acceptable clinical outcome as indicated by researches. However, these previous clinical studies involved patients strictly following inclusion criteria. In real-world situations, where the situation of patients is more complicated, the ecacy of salvage treatment of Olaparib with or without abiraterone-prednisone remains to be unclear. Case presentation: The present case displayed a 61-year-old man who was initially diagnosed with metastatic hormone-sensitive prostate cancer(mHSPC) and proceeding into mCRPC after several kinds of standard therapies. Surprisingly, the patient had a well TPSA response and remission of symptoms for four months by taking Olaparib combined with abiraterone-prednisone, basing on androgen-deprivation therapy (ADT). The resistance of Olaparib was occurred with slowly increasing serum TPSA level again. -

CROSSE FIT Utah Lacrosse Blazes a New Trail As a Division I Program
SPRING 2019 CROSSE FIT Utah lacrosse blazes a new trail as a Division I program. CHOOSING A MAJOR DOCTORS WITH RURAL ROOTS PRESERVING DARK SKIES 150TH COMMENCEMENT SPRING 2019 VOL. 28 NO. 4 34 Crosse Fit Utah lacrosse blazes a new trail as a Division I program. 14 Dark Skies Utah’s untapped natural resource. 18 Rural Roots U medical students visit remote high schools around the state. 34 26 Major Decisions Tips for choosing a major and navigating the academic journey. DEPARTMENTS 2 Feedback 4 Campus Scene 6 Updates 10 Bookshelf 12 Discovery 40 Alum News 48 One More Cover photo of U Lacrosse goalie Liam Donnelly 18 14 by Dave Titensor 26 4 FEEDBACK Kerry Peterson The body donation Publisher people [at the U] I William Warren dealt with were all Editor-in-Chief very respectful and J. Melody Murdock honestly some of the Art Director/Photographer most kind and under- David E. Titensor BFA’91 standing that I have Managing Editor ever met. They were Seth Bracken truthful and honest Senior Editor Marcia C. Dibble and answered every Business Manager question that we had. Brian Rasmussen BA’80 It was also a really Corporate Sponsors nice weekend this ARUP Laboratories Intermountain Healthcare his dedication to and coordinated last summer as the Nationwide the integrity of the multiple cadaver labs new names that were University Credit Union University of Utah Health Plans program was very for [the U’s] surgeons etched into the wall of reassuring. I will in training. I see the panels were unveiled. Editorial Advisory Committee Joe Borgenicht share this article with reverence in which Neelam Chand BS’08 Andy Cier my family to help bodies are handled, Steven Martinez BS’82 Debra Daniels MSW’84 them understand the the respectful Salt Lake City Greta Belanger deJong Peter Esko BA’03 I will share this importance of my language that is used, Lindsey Ferrari decision. -

Furthering National Development Through Sport, the Case of Qatar Wadih Ishac
Furthering national development through sport, the case of Qatar Wadih Ishac To cite this version: Wadih Ishac. Furthering national development through sport, the case of Qatar. Business administra- tion. Université Bourgogne Franche-Comté, 2018. English. NNT : 2018UBFCH037. tel-02071444 HAL Id: tel-02071444 https://tel.archives-ouvertes.fr/tel-02071444 Submitted on 18 Mar 2019 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. UNIVERSITE DE BOURGOGNE UFR des Sciences et Techniques des Activités Physiques et Sportives THÈSE Pour obtenir le grade de Docteur de l’Université de Bourgogne Discipline : Sciences et Techniques des Activités Physiques et Sportives Par ISHAC Wadih August 2018 Furthering national development through sport, the case of Qatar Directeur de thèse : BOUCHET Patrick, Professeur des Universités, Université de Bourgogne Franche-Comté Co-directeur de thèse : SOBRY Claude, Professeur des Universités, Université de Lille 2 Jury : Président : Mohammed Kaach, Professeur, Institut National des Sports Moulay Rachid Rapporteurs : Ati Abdessatar Professeur, Jazan University Kaouther Kooli Professeur, Bournemouth University Sorina Cernaianu,Professeur, University of Craiova Win if you can Lose if you must But never QUIT Cameron Trammell Declaration This dissertation is the result of my own work and includes nothing which is the outcome of work done in collaboration, except where specifically indicated in the text.