Environmental Change and Its Impact on Hybridising Daphnia Species Complexes
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Norwegian Hydrological Reference Dataset for Climate Change Studies
Norwegian Hydrological Reference Dataset for Climate Change Studies Anne K. Fleig (Ed.) 2 2013 RAPPORT Norwegian Hydrological Reference Dataset for Climate Change Studies Norwegian Water Resources and Energy Directorate 2013 Report no. 2 – 2013 Norwegian Hydrological Reference Dataset for Climate Change Studies Published by: Norwegian Water Resources and Energy Directorate Editor: Anne K. Fleig Authors: Anne K. Fleig, Liss M. Andreassen, Emma Barfod, Jonatan Haga, Lars Egil Haugen, Hege Hisdal, Kjetil Melvold, Tuomo Saloranta Print: Norwegian Water Resources and Energy Directorate Number printed: 50 Femundsenden, spring 2000, Photo: Vidar Raubakken and Cover photo: Gunnar Haugen, NVE. ISSN: 1501-2832 ISBN: 978-82-410-0869-6 Abstract: Based on the Norwegian hydrological measurement network, NVE has selected a Hydrological Reference Dataset for studies of hydrological change. The dataset meets international standards with high data quality. It is suitable for monitoring and studying the effects of climate change on the hydrosphere and cryosphere in Norway. The dataset includes streamflow, groundwater, snow, glacier mass balance and length change, lake ice and water temperature in rivers and lakes. Key words: Reference data, hydrology, climate change Norwegian Water Resources and Energy Directorate Middelthunsgate 29 P.O. Box 5091 Majorstua N 0301 OSLO NORWAY Telephone: +47 22 95 95 95 Fax: +47 22 95 90 00 E-mail: [email protected] Internet: www.nve.no January 2013 Contents Preface ................................................................................................ -

Fylkesmannens Tilrådning Frivillig Skogvern Og Vern På Statskog 2019
Fylkesmannens tilrådning Frivillig skogvern og vern på Statskog 2019 Mefosselva - Flatanger kommune Honnavasslia - utvidelse, Flatanger kommune Storvatnet - Namdalseid kommune Hjartvikfjellet - Namdalseid kommune Gøllaustjønna og Langdalen - Namdalseid kommune Husåstjønnbekken - Namdalseid kommune Finnsåsmarka - utvidelse Snåsa kommune Bårvassåsen - Indre Fosen kommune Raudkamlia - Indre Fosen kommune Skjettenberglia - utvidelse, Indre Fosen kommune Vargøylia - Indre Fosen kommune Trongstadlia - Åfjord kommune Henfallet - utvidelse Tydal kommune Stavåa - utvidelse Rennebu Storvika - utvidelse Selbu kommune Vuddudalen – Levanger kommune Mariafjellet – Skardbekken/ Tjaetsiegaske - utvidelse Lierne Tjuvdalen, utvidelse av Blåfjella-Skjækerfjella/Låarte-Skæhkere nasjonalpark, Verdal kommune Fylkesmannen i Trøndelag August 2019 Innhold 1. FORSLAG.............................................................................................................................................. 4 1.1. Hjemmelsgrunnlag og bakgrunn for vernet ................................................................................. 4 1.2. Verneverdier, påvirkningsfaktorer og effekter av verneforslaget ............................................... 5 1.3. Andre interesser........................................................................................................................... 7 1.4. Planstatus ..................................................................................................................................... 7 2. SAKSBEHANDLING -

Sjørøyevassdragene I Nord-Norge; 100 Av 400 Mulige
UTREDNING DN-utredning 1-2012 Sjørøyevassdragene i Nord-Norge; 100 av 400 mulige – en zoogeografisk analyse av de aktuelle vassdragene Sjørøyevassdragene i Nord-Norge; 100 av 400 mulige – en zoogeografisk analyse av de aktuelle vassdragene DN-utredning 1-2012 EKSTRAKT: ABSTRACT: Kartleggingen av vel 400 nord-norske Mapping of more than 400 watercourses Utgiver: vassdrag viser at det er ca 100 sjørøye- in Northern Norway, shows that there are Direktoratet for naturforvaltning vassdrag i landsdelen. Undersøkelsene viser populations of anadromous Arctic char sam tidig at sjørøya blir stadig viktigere in about 100 of them. The investigations Dato: Mars 2012 når en beveger seg nordover, og helt nord also show that anadromous char becomes i landet er det mye sjørøye og svært lite more usual the further north you move. In Antall sider: 36 sjøørret. I Nordland er det omtrent bare Nordland county, almost all populations of Emneord: innsjø baserte bestander, mens det i Nord- anadromous char are lake-based while in Sjørøye Troms og Finnmark i tillegg er en del elve- North-Troms and Finnmark there are also Laks baserte bestander. Andelen individer som a number of river-based populations. The Sjøørret vandrer (i enhver populasjon), øker også part of the population which migrates to Sjøvandring når en beveger seg nordover. I rapporten sea, also increases as you move north. The Nord-Norge blir årsakene til de observerte tendensene report discusses the reasons behind the diskutert. observed differences. Keywords: Anadromous Arctic char Atlantic salmon Brown trout Sea-migration North-Norway Bestilling: Direktoratet for naturforvaltning, postboks 5672 Sluppen, 7485 Trondheim Telefon: 73 58 05 00 Telefaks: 73 58 05 01 www.dirnat.no/publikasjoner Refereres som: Halvorsen, M. -

MIAMI UNIVERSITY the Graduate School Certificate for Approving The
MIAMI UNIVERSITY The Graduate School Certificate for Approving the Dissertation We hereby approve the Dissertation of Sandra J. Connelly Candidate for the Degree: Doctor of Philosophy __________________________________________ Director Dr. Craig E. Williamson __________________________________________ Reader Dr. Maria González __________________________________________ Reader Dr. David L. Mitchell __________________________________________ Graduate School Representative Dr. A. John Bailer ABSTRACT EFFECTS OF ULTRAVIOLET RADIATION (UVR) INDUCED DNA DAMAGE AND OTHER ECOLOGICAL DETERMINANTS ON CRYPTOSPORIDIUM PARVUM, GIARDIA LAMBLIA, AND DAPHNIA SPP. IN FRESHWATER ECOSYSTEMS Sandra J. Connelly Freshwater ecosystems are especially susceptible to climatic change, including anthropogenic-induced changes, as they are directly influenced by the atmosphere and terrestrial ecosystems. A major environmental factor that potentially affects every element of an ecosystem, directly or indirectly, is ultraviolet radiation (UVR). UVR has been shown to negatively affect the DNA of aquatic organisms by the same mechanism, formation of photoproducts (cyclobutane pyrimidine dimers; CPDs), as in humans. First, the induction of CPDs by solar UVR was quantified in four aquatic and terrestrial temperate ecosystems. Data show significant variation in CPD formation not only between aquatic and terrestrial ecosystems but also within a single ecosystem and between seasons. Second, there is little quantitative data on UV-induced DNA damage and the effectiveness of DNA repair mechanisms on the damage induced in freshwater invertebrates in the literature. The rate of photoproduct induction (CPDs) and DNA repair (photoenzymatic and nucleotide excision repair) in Daphnia following UVR exposures in artificial as well as two natural temperate lake systems was tested. The effect of temperature on the DNA repair rates, and ultimately the organisms’ survival, was tested under controlled laboratory conditions following artificial UVB exposure. -

Methods for Selection of Daphnia Resting Eggs: the Influence of Manual Decapsulation and Sodium Hypoclorite Solution on Hatching Rates T
http://dx.doi.org/10.1590/1519-6984.09415 Original Article Methods for selection of Daphnia resting eggs: the influence of manual decapsulation and sodium hypoclorite solution on hatching rates T. A. S. V. Paesa, A. C. Rietzlera and P. M. Maia-Barbosaa* aPostgraduate Programme in Ecology, Conservation and Management of Wildlife – ECMVS, Laboratory of Limnology, Ecotoxicology and Aquatic Ecology, Universidade Federal de Minas Gerais – UFMG, Av. Antônio Carlos, 6627, Pampulha, CEP 31270-901, Belo Horizonte, MG, Brazil *e-mail: [email protected] Received: June 19, 2015 – Accepted: October 7, 2015 – Distributed: November 30, 2016 (Wtih 4 figures) Abstract Cladocerans are able to produce resting eggs inside a protective resistant capsule, the ephippium, that difficults the visualization of the resting eggs, because of the dark pigmentation. Therefore, before hatching experiments, methods to verify viable resting eggs in ephippia must be considered. This study aimed to evaluate the number of eggs per ephippium of Daphnia from two tropical aquatic ecosystems and the efficiency of some methods for decapsulating resting eggs. To evaluate the influence of methods on hatching rates, three different conditions were tested: immersion in sodium hypochlorite, manually decapsulated resting eggs and intact ephippia. The immersion in hypochlorite solution could evaluate differences in numbers of resting eggs per ephippium between the ecosystems studied. The exposure to sodium hypochlorite at a concentration of 2% for 20 minutes was the most efficient method for visual evaluation and isolation of the resting eggs. Hatching rate experiments with resting eggs not isolated from ephippia were underestimated (11.1 ± 5.0%), showing the need of methods to quantify and isolate viable eggs. -

(Med Hensyn På Sjøvandring) I Dønna, Ofoten, Lofoten Og Vesterålen
Rapport 2008-05 Kartlegging av fiskebestander med usikker bestandsstatus (med hensyn på sjøvandring) i Dønna, Ofoten, Lofoten og Vesterålen Nordnorske Ferskvannsbiologer Sortland Kartlegging av bestander med usikker bestandsstus i Nordland 2008 Rapport nr. 2008-05 Antall sider: 110 Tittel : Kartlegging av fiskebestander med usikker bestandsstatus (med hensyn på sjøvandring) i Dønna, Ofoten, Lofoten og Vesterålen Forfatter : Morten Halvorsen og Lisbeth Jørgensen Oppdragsgiver : Fylkesmannen i Nordland Sammendrag: Resultater fra vassdrag der innsjøene ble prøvefisket: Kommune Vassdrag Innsjø Materiale Antall Andel (%) ørret sjøfisk Bø Røsnesvassdraget. Røsnesvatn 60 2 3 Bø Straumevassdraget Langvatn vest 96 0 0 Hadsel Flatsetvassdraget Flatsetvatn 66 11 17 Hadsel Kaljordvassdraget Kaljordvatnet 107 12 11 Hadsel Breivikvassdraget Dalvatnet 83 26 31 Sortland Lakselva i Godfjorden Eidesvatna - - - Sortland Selnesvassdraget Selnesvatnet 75 36 48 Sortland Holmstadvassdraget Durmålsvatnet 70 12 17 Sortland Langvatnvassdraget Langvatnet 89 4 5 Øksnes Nordsandvassdraget Storvatnet/Nedrevatn 40 14 35 ” ” Øvrevatn 36 11 31 Øksnes Grunnvatnvassdraget Grunnvatnet 100 32 32 Øksnes Urdskardvassdraget Kjørvatn (Nedrevatn) 34 24 71 ” ” Sennvatn 47 22 46 Lødingen Saltvatnvassdraget Saltvatnet 93 27 29 Vestvågøy Storfjordvassdraget Nedre Storfjordvatn 96 6 6 ” ” Øvre Storfjordvatn 60 0 0 Vestvågøy Torvdalsvassdraget Lille Torvdalsvatnet 30 4 13 ” ” Store Torvdalsvatnet 58 6 10 Vestvågøy Vestresandvassdraget Urdvatnet/Haukelandsvatn 50 0 0 Vestvågøy Helos/Lyngedalsvassdraget -

Forekomst Av Reproduserende Bestander Av Bekke- Røye (Salvelinus Fontinalis) I Norge Pr
Forekomst av reproduserende bestander av bekke- røye (Salvelinus fontinalis) i Norge pr. 2013 Trygve Hesthagen og Einar Kleiven NINAs publikasjoner NINA Rapport Dette er en elektronisk serie fra 2005 som erstatter de tidligere seriene NINA Fagrapport, NINA Oppdragsmelding og NINA Project Report. Normalt er dette NINAs rapportering til oppdragsgiver etter gjennomført forsknings-, overvåkings- eller utredningsarbeid. I tillegg vil serien favne mye av instituttets øvrige rapportering, for eksempel fra seminarer og konferanser, resultater av eget forsk- nings- og utredningsarbeid og litteraturstudier. NINA Rapport kan også utgis på annet språk når det er hensiktsmessig. NINA Temahefte Som navnet angir behandler temaheftene spesielle emner. Heftene utarbeides etter behov og se- rien favner svært vidt; fra systematiske bestemmelsesnøkler til informasjon om viktige problemstil- linger i samfunnet. NINA Temahefte gis vanligvis en populærvitenskapelig form med mer vekt på illustrasjoner enn NINA Rapport. NINA Fakta Faktaarkene har som mål å gjøre NINAs forskningsresultater raskt og enkelt tilgjengelig for et større publikum. De sendes til presse, ideelle organisasjoner, naturforvaltningen på ulike nivå, politikere og andre spesielt interesserte. Faktaarkene gir en kort framstilling av noen av våre viktigste forsk- ningstema. Annen publisering I tillegg til rapporteringen i NINAs egne serier publiserer instituttets ansatte en stor del av sine viten- skapelige resultater i internasjonale journaler, populærfaglige bøker og tidsskrifter. Forekomst -

Frostating Lagmannsrett - LF-2016-17908 - LF-2016-17920
Utskrift fra Lovdata - 02.08.2016 09:24 Frostating lagmannsrett - LF-2016-17908 - LF-2016-17920 Instans Frostating lagmannsrett - Dom Dato 2016-07-05 Publisert LF-2016-17908 - LF-2016-17920 Stikkord Utslippstillatelse. Drikkevannskilde. Forurensning. Reell forurensningsfare. Forurensningsloven § 7 og § 18 fjerde ledd. Forvaltningsloven § 24 og § 25. Sammendrag Spørsmål om gyldigheten av en kommunes tilbakekallelse av utslippstillatelser fra hytter, begrunnet i hensynet til sikkert drikkevann (Jonsvatnet i Trondheim). Kommunens vedtak ble opprettholdt av lagmannsretten, både materielt og prosessuelt. Faren for forurensning var stor for den ene eiendommen, og reell også for den andre, som lå 550 meter fra Jonsvatnet. Drøftelse av forvaltningens kompetanse etter forurensningsloven § 18 tredje ledd og av domstolens prøvingskompetanse. Saksgang Sør-Trøndelag tingrett TSTRO-2015-61931 - Frostating lagmannsrett LF-2016- 17908 / LF-2016-17920 (16-017908ASD-FROS / 16-017920ASD-FROS). Parter R-O Holding AB og Tore Haagensli (for begge: advokat Jostein Grosås), mot Trondheim kommune (advokat Sverre Bernt Schistad). Forfatter Førstelagmann Sverre Erik Jebens. Førstelagmann Sven-Jørgen Lindsetmo. Førstelagmann Willy Nesset. LF-2016-17908 - LF-2016-17920 Side 1 Utskrift fra Lovdata - 02.08.2016 09:24 Saken gjelder tvist om gyldigheten av et kommunalt vedtak om tilbakekall av utslippstillatelse i ferskvann, nærmere bestemt pålegg om fjerning eller plugging av innvendig vannledning til fritidsboliger. Jonsvatnet, som ligger noe sør for Trondheim sentrum, er primær vannforsyningskilde for Trondheim kommune og reservevannkilde for Melhus kommune. Innsjøen forsyner ca 200 000 personer med vann, og er også vannkilde for en rekke virksomheter, herunder St. Olavs Hospital i Trondheim. Jonsvatnet er den største innsjøen innenfor Trondheim kommunes grenser, og består av tre sammenhengende deler; Storvatnet, Litjvatnet og Kilvatnet. -

Taxonomic Atlas of the Water Fleas, “Cladocera” (Class Crustacea) Recorded at the Old Woman Creek National Estuarine Research Reserve and State Nature Preserve, Ohio
Taxonomic Atlas of the Water Fleas, “Cladocera” (Class Crustacea) Recorded at the Old Woman Creek National Estuarine Research Reserve and State Nature Preserve, Ohio by Jakob A. Boehler, Tamara S. Keller and Kenneth A. Krieger National Center for Water Quality Research Heidelberg University Tiffin, Ohio, USA 44883 January 2012 Taxonomic Atlas of the Water Fleas, “Cladocera” (Class Crustacea) Recorded at the Old Woman Creek National Estuarine Research Reserve and State Nature Preserve, Ohio by Jakob A. Boehler, Tamara S. Keller* and Kenneth A. Krieger Acknowledgements The authors are grateful for the assistance of Dr. David Klarer, Old Woman Creek National Estuarine Research Reserve, for providing funding for this project, directing us to updated taxonomic resources and critically reviewing drafts of this atlas. We also thank Dr. Brenda Hann, Department of Biological Sciences at the University of Manitoba, for her thorough review of the final draft. This work was funded under contract to Heidelberg University by the Ohio Department of Natural Resources. This publication was supported in part by Grant Number H50/CCH524266 from the Centers for Disease Control and Prevention. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of Centers for Disease Control and Prevention. The Old Woman Creek National Estuarine Research Reserve in Ohio is part of the National Estuarine Research Reserve System (NERRS), established by Section 315 of the Coastal Zone Management Act, as amended. Additional information about the system can be obtained from the Estuarine Reserves Division, Office of Ocean and Coastal Resource Management, National Oceanic and Atmospheric Administration, U.S. -
3323 72Dpi.Pdf (329.1Kb)
1 Norsk institutt for vannforskning O-91050 Landsomfattende trofiundersøkelse av innsjøer Problemnotat om tilfeldig utvalg av innsjøer 1 FORORD Bakgrunnen for dette notatet var diskusjoner i SFT og NIVA høsten 1994 om behovet for at innsjøer i landsomfattende undersøkelser skal trekkes ut statistisk tilfeldig for å tilfredsstille de aktuelle målsetninger med undersøkelsene. Diskusjonene har gått parallelt for "Landsomfattende trofiundersøkelse av norske innsjøer" og "1000-sjøer undersøkelsen av forsuring". Sistnevnte skal gjennomføres på nytt i 1995, og det er planer om å utvide antallet innsjøer som skal undersøkes. Da målsettingen med de to undersøkelsene er noe forskjellig - og ikke minst fordi de fenomenene en skulle studere var ulikt fordelt over landet, ble det også diskutert om strategien for utvalg av innsjøer kan/bør være forskjellig. For "trofiundersøkelsen" ble det avholdt et diskusjonsmøte i SFT den 18. januar 1995. Møtet konkluderte med at det er hensiktsmessig å fortsette undersøkelsen med det utvalget av innsjøer som ble gjort i 1988, med enkelte tillegg i 1992. Det var også enighet om behovet for å utarbeide et notat med presentasjon av endel synspunkter på tilfeldig utvalg av innsjøer. Synspunktene representerer primært de sider av problematikken som er relevante for trofiundersøkelsen, og er ikke nødvendigvis dekkende for andre undersøkelser. Gunnar Severinsen har tilrettelagt data fra Vassdragsregisteret og bidratt ved bearbeidingen av disse. Oslo 31. mai 1995 Bjørn Faafeng 2 INNHOLD side FORORD 1 INNHOLD 2 1. KONKLUSJONER 3 2. TILFELDIG UTVALG 4 2.1 Definisjon og utvalg 4 2.2 Stratifisert tilfeldig utvalg 4 2.3 Tilfeldig utvalg eller ikke? - målsetting og rammebetingelser avgjør! 5 3. -

A New Species in the Daphnia Curvirostris (Crustacea: Cladocera)
JOURNAL OF PLANKTON RESEARCH VOLUME 28 NUMBER 11 PAGES 1067–1079 2006 j j j j A new species in the Daphnia curvirostris (Crustacea: Cladocera) complex from the eastern Palearctic with molecular phylogenetic evidence for the independent origin of neckteeth ALEXEY A. KOTOV1*, SEIJI ISHIDA2 AND DEREK J. TAYLOR2 1 2 A. N. SEVERTSOV INSTITUTE OF ECOLOGY AND EVOLUTION, LENINSKY PROSPECT 33, MOSCOW 119071, RUSSIA AND DEPARTMENT OF BIOLOGICAL SCIENCES, UNIVERSITY AT BUFFALO, THE STATE UNIVERSITY OF NEW YORK, BUFFALO, NY 14260, USA *CORRESPONDING AUTHOR: [email protected] Received February 22, 2006; accepted in principle June 22, 2006; accepted for publication August 31, 2006; published online September 8, 2006 Communicating editor: K.J. Flynn Little is known of the biology and diversity of the environmental model genus Daphnia beyond the Nearctic and western Palearctic. Here, we describe Daphnia sinevi sp. nov., a species superficially similar to Daphnia curvirostris Eylmann, 1878, from the Far East of Russia. We estimated its phylogenetic position in the subgenus Daphnia s. str. with a rapidly evolving mitochondrial protein coding gene [NADH-2 (ND2)] and a nuclear protein-coding gene [heat shock protein 90 (HSP90)]. Daphnia curvirostris, D. sinevi sp. nov., Daphnia tanakai and D. sp. from Ootori- Ike, Japan, (which, probably, is D. morsei Ishikawa, 1895) formed a monophyletic clade modestly supported by ND2 and strongly supported by HSP90. Our results provide evidence of hidden species diversity in eastern Palearctic Daphnia, independent origins of defensive neckteeth and phylogenetic informativeness of nuclear protein-coding genes for zooplankton genera. INTRODUCTION morphological and genetic approach, Ishida et al. -
Jordarter V E U N O T N a Leirpollen
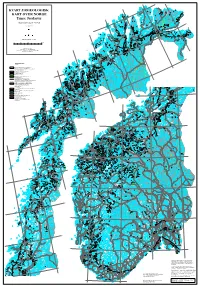
30°E 71°N 28°E Austhavet Berlevåg Bearalváhki 26°E Mehamn Nordkinnhalvøya KVARTÆRGEOLOGISK Båtsfjord Vardø D T a e n a Kjøllefjord a n f u j o v r u d o e Oksevatnet t n n KART OVER NORGE a Store L a Buevatnet k Geatnjajávri L s Varangerhalvøya á e Várnjárga f g j e o 24°E Honningsvåg r s d Tema: Jordarter v e u n o t n a Leirpollen Deanodat Vestertana Quaternary map of Norway Havøysund 70°N en rd 3. opplag 2013 fjo r D e a T g tn e n o a ra u a a v n V at t j j a n r á u V Porsanger- Vadsø Vestre Kjæsvatnet Jakobselv halvøya o n Keaisajávri Geassájávri o Store 71°N u Bordejávrrit v Måsvatn n n i e g Havvannet d n r evsbotn R a o j s f r r Kjø- o Bugøy- e fjorden g P fjorden 22°E n a Garsjøen Suolo- s r Kirkenes jávri o Mohkkejávri P Sandøy- Hammerfest Hesseng fjorden Rypefjord t Bjørnevatn e d n Målestokk (Scale) 1:1 mill. u Repparfjorden s y ø r ø S 0 25 50 100 Km Sørøya Sør-Varanger Sállan Skáiddejávri Store Porsanger Sametti Hasvik Leaktojávri Kartet inngår også i B áhèeveai- NASJONALATLAS FOR NORGE 20°E Leavdnja johka u a Lopphavet