US 2014/0057832 A1 Conrad Et Al
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Endothelin System and Therapeutic Application of Endothelin Receptor
xperim ACCESS Freely available online & E en OPEN l ta a l ic P in h l a C r m f o a c l a o n l o r g u y o J Journal of ISSN: 2161-1459 Clinical & Experimental Pharmacology Research Article Endothelin System and Therapeutic Application of Endothelin Receptor Antagonists Abebe Basazn Mekuria, Zemene Demelash Kifle*, Mohammedbrhan Abdelwuhab Department of Pharmacology, School of Pharmacy, College of Medicine and Health Sciences, University of Gondar, Gondar, Ethiopia ABSTRACT Endothelin is a 21 amino acid molecule endogenous potent vasoconstrictor peptide. Endothelin is synthesized in vascular endothelial and smooth muscle cells, as well as in neural, renal, pulmonic, and inflammatory cells. It acts through a seven transmembrane endothelin receptor A (ETA) and endothelin receptor B (ETB) receptors belongs to G protein-coupled rhodopsin-type receptor superfamily. This peptide involved in pathogenesis of cardiovascular disorder like (heart failure, arterial hypertension, myocardial infraction and atherosclerosis), renal failure, pulmonary arterial hypertension and it also involved in pathogenesis of cancer. Potentially endothelin receptor antagonist helps the treatment of the above disorder. Currently, there are a lot of trails both per-clinical and clinical on endothelin antagonist for various cardiovascular, pulmonary and cancer disorder. Some are approved by FAD for the treatment. These agents are including both selective and non-selective endothelin receptor antagonist (ETA/B). Currently, Bosentan, Ambrisentan, and Macitentan approved -

A2) United States Patent (10) Patent No.: US 8,940,711 B2 Olsonet Al
US008940711B2 a2) United States Patent (10) Patent No.: US 8,940,711 B2 Olsonet al. (45) Date of Patent: Jan. 27, 2015 (54) MICRO-RNA FAMILY THAT MODULATES C1I2N 2310/321 (2013.01); C12N 2310/346 FIBROSIS AND USES THEREOF (2013.01); CI2N 2310/3515 (2013.01); C12N (71) Applicant: The Board of Regents, The University 2320/31 (2013.01); CI2N 2330/10 (2013.01) of Texas System, Austin, TX (US) USPC iececcsesseeseeesenensceesensessecsenesenscnseeee 514/444 (58) Field of Classification Search (72) Inventors: Erie N. Olson, Dallas, TX (US); Eva USPC iececcsesseeseeesenensceesensessecsenesenscnseeee 514/44A van Rooij, Utrecht (NL) See application file for complete search history. (56) References Cited (73) Assignee: The Board of Regents, The University of Texas System, Austin, TX (US) U.S. PATENT DOCUMENTS Notice: Subject to any disclaimer, the term of this 7,232,806 B2 6/2007 Tuschletal. (*) 7,674,617 B2 3/2010 Kim etal. patent is extended or adjusted under 35 2005/0059005 Al 3/2005 Tuschletal. U.S.C. 154(b) by 1 day. 2005/0222399 Al 10/2005 Bentwich 2005/0261218 Al 11/2005 Esau etal. (21) Appl. No.: 13/840,242 2006/0019286 Al 1/2006 Horvitz et al. 2006/0105360 Al 5/2006 Croceet al. Filed: Mar.15, 2013 2006/0185027 Al 8/2006 Bartel etal. (22) 2006/0247193 Al 11/2006 Taira etal. 2007/0087335 Al 4/2007 Brahmacharietal. (65) Prior Publication Data 2007/0092882 Al 4/2007 Wangetal. US 2013/0261169 Al Oct. 3, 2013 2008/0050722 Al 2/2008 Kim etal. 2008/0176766 Al 7/2008 Brown etal. -
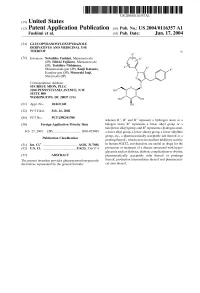
(12) Patent Application Publication (10) Pub. No.: US 2004/0116357 A1 Fushimi Et Al
US 2004O116357A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2004/0116357 A1 Fushimi et al. (43) Pub. Date: Jun. 17, 2004 (54) GLUCOPYRANOSYLOXYPYRAZOLE DERVATIVES AND MEDICINAL USE THEREOF (I) (76) Inventors: Nobuhiko Fushimi, Matsumoto-shi (JP); Hideki Fujikura, Matsumoto-shi (JP); Toshihiro Nishimura, Minamiazumi-gun (JP); Kenji Katsuno, Kamiina-gun (JP); Masayuki Isaji, Shiojiri-shi (JP) Correspondence Address: SUGHRUE MION, PLLC 2100 PENNSYLVANIAAVENUE, N.W. SUTE 800 WASHINGTON, DC 20037 (US) (21) Appl. No.: 10/469,140 (22) PCT Filed: Feb. 26, 2002 (86) PCT No.: PCT/JP02/01708 wherein R', R and R represent a hydrogen atom or a (30) Foreign Application Priority Data halogen atom; R" represents a lower alkyl group or a halo(lower alkyl) group; and R represents a hydrogen atom, Feb. 27, 2001 (JP)...................................... 2001-053085 a lower alkyl group, a lower alkoxy group, a lower alkylthio group, etc., a pharmaceutically acceptable Salt thereof or a Publication Classification prodrug thereof., which exert an excellent inhibitory activity (51) Int. Cl. ................................................ A61K 31/7056 in human SGLT2, and therefore are useful as drugs for the (52) U.S. Cl. ............................................. 514/23: 536/17.4 prevention or treatment of a disease associated with hyper glycemia Such as diabetes, diabetic complications or obesity, (57) ABSTRACT pharmaceutically acceptable Salts thereof or prodrugs The present invention provides glucopyranosyloxypyrazole thereof, production intermediates thereof and pharmaceuti derivatives represented by the general formula: cal uses thereof. US 2004/0116357 A1 Jun. 17, 2004 GLUCOPYRANOSYLOXYPYRAZOLE 0005. In recent years, development of new type antidia DERVATIVES AND MEDICINAL USE THEREOF betic agents has been progressing, which promote urinary glucose excretion and lower blood glucose level by prevent TECHNICAL FIELD ing excess glucose reabsorption at the kidney (J. -

Supplementary Information
Supplementary Information Network-based Drug Repurposing for Novel Coronavirus 2019-nCoV Yadi Zhou1,#, Yuan Hou1,#, Jiayu Shen1, Yin Huang1, William Martin1, Feixiong Cheng1-3,* 1Genomic Medicine Institute, Lerner Research Institute, Cleveland Clinic, Cleveland, OH 44195, USA 2Department of Molecular Medicine, Cleveland Clinic Lerner College of Medicine, Case Western Reserve University, Cleveland, OH 44195, USA 3Case Comprehensive Cancer Center, Case Western Reserve University School of Medicine, Cleveland, OH 44106, USA #Equal contribution *Correspondence to: Feixiong Cheng, PhD Lerner Research Institute Cleveland Clinic Tel: +1-216-444-7654; Fax: +1-216-636-0009 Email: [email protected] Supplementary Table S1. Genome information of 15 coronaviruses used for phylogenetic analyses. Supplementary Table S2. Protein sequence identities across 5 protein regions in 15 coronaviruses. Supplementary Table S3. HCoV-associated host proteins with references. Supplementary Table S4. Repurposable drugs predicted by network-based approaches. Supplementary Table S5. Network proximity results for 2,938 drugs against pan-human coronavirus (CoV) and individual CoVs. Supplementary Table S6. Network-predicted drug combinations for all the drug pairs from the top 16 high-confidence repurposable drugs. 1 Supplementary Table S1. Genome information of 15 coronaviruses used for phylogenetic analyses. GenBank ID Coronavirus Identity % Host Location discovered MN908947 2019-nCoV[Wuhan-Hu-1] 100 Human China MN938384 2019-nCoV[HKU-SZ-002a] 99.99 Human China MN975262 -

Deliverable 5.A Interim Report on the Study Results APPENDIX 2
Deliverable 5.a Interim report on the study results APPENDIX 2: Algorithms used to identify study variables for service contract EMA/2011/38/CN ‐ PIOGLITAZONE November 28th 2012 D5.a Interim report on the study results (Appendix 2) for Service Contract EMA/2011/38/CN PIOGLITAZONE Author(s): Vera Ehrenstein (AUH‐AS) APPENDIX 2. ALGORITHMS USED TO IDENTIFY STUDY VARIABLES Algorithms for AU Database DISEASE/CONDITION ICD-8 CODE (1977-1993) ICD-10 CODE (1994-) Diabetes type 2 250.00; 250.06; 250.07; 250.09 E11.0; E11.1; E11.9 Cancer of bladder 188 C67 Haematuria N/A R31 Haematuria, unspecified B18, K70.0–K70.3, K70.9, K71, K73, Mild hepatic impairment 571, 573.01, 573.04 K74, K76.0 Moderate to severe hepatic 070.00, 070.02, 070.04, 070.06, B15.0, B16.0, B16.2, B19.0, K70.4, impairment 070.08, 573.00, 456.00–456.09 K72, K76.6, I85 Acute myocardial infarction 410 I21-I23 Acute coronary syndrome 410, 413 I20-I24 Ischemic heart disease 410-414 I20-I25 427.09, 427.10, 427.11, 427.19, Congestive heart failure I50, I11.0, I13.0,I13.2 428.99, 782.49; Acute renal failure N/A N17 Diabetic coma N/A E10.0, E11.0, E12.0,E13.0, E14.0 Diabetic acidosis N/A E10.1, E11.1, E12.1,E13.1, E14.1 F10.1-F10.9, G31.2, G62.1, G72.1, Alcoholism 291, 303, 577.10, 571.09, 571.10 I42.6, K29.2, K86.0, Z72.1 Obesity 277.99 E65-E66 D5.a Interim report on the study results (Appendix 2) for Service Contract EMA/2011/38/CN PIOGLITAZONE Author(s): Vera Ehrenstein (AUH‐AS) Algorithms for defining acute events in Denmark, ICD-10 code Event ICD-10 code I21.x, I23.x http://apps.who.int/classifications/icd10/browse/2010/en#/I21 -

Title 16. Crimes and Offenses Chapter 13. Controlled Substances Article 1
TITLE 16. CRIMES AND OFFENSES CHAPTER 13. CONTROLLED SUBSTANCES ARTICLE 1. GENERAL PROVISIONS § 16-13-1. Drug related objects (a) As used in this Code section, the term: (1) "Controlled substance" shall have the same meaning as defined in Article 2 of this chapter, relating to controlled substances. For the purposes of this Code section, the term "controlled substance" shall include marijuana as defined by paragraph (16) of Code Section 16-13-21. (2) "Dangerous drug" shall have the same meaning as defined in Article 3 of this chapter, relating to dangerous drugs. (3) "Drug related object" means any machine, instrument, tool, equipment, contrivance, or device which an average person would reasonably conclude is intended to be used for one or more of the following purposes: (A) To introduce into the human body any dangerous drug or controlled substance under circumstances in violation of the laws of this state; (B) To enhance the effect on the human body of any dangerous drug or controlled substance under circumstances in violation of the laws of this state; (C) To conceal any quantity of any dangerous drug or controlled substance under circumstances in violation of the laws of this state; or (D) To test the strength, effectiveness, or purity of any dangerous drug or controlled substance under circumstances in violation of the laws of this state. (4) "Knowingly" means having general knowledge that a machine, instrument, tool, item of equipment, contrivance, or device is a drug related object or having reasonable grounds to believe that any such object is or may, to an average person, appear to be a drug related object. -
Guidance for the Format and Content of the Protocol of Non-Interventional
PASS information Title Metformin use in renal impairment Protocol version identifier Version 2 Date of last version of 30 October 2013 protocol EU PAS register number Study not registered Active substance A10BA02 metformin Medicinal product Metformin Product reference N/A Procedure number N/A Marketing authorisation 1A Farma, Actavis, Aurobindo, Biochemie, Bluefish, holder(s) Hexal, Mylan, Orifarm, Pfizer, Sandoz, Stada, Teva Joint PASS No Research question and To assess the use and safety of metformin in patients objectives with and without renal insufficiency in current clinical practice in at least two EU Member States. Country(-ies) of study Denmark, United Kingdom Author Christian Fynbo Christiansen, MD, PhD Page 1/214 Marketing authorisation holder(s) Marketing authorisation N/A holder(s) MAH contact person N/A Page 2/214 1. Table of Contents PASS information .......................................................................................................... 1 Marketing authorisation holder(s) .................................................................................... 2 1. Table of Contents ...................................................................................................... 3 2. List of abbreviations ................................................................................................... 4 3. Responsible parties .................................................................................................... 5 4. Abstract .................................................................................................................. -

(12) United States Patent )-X- NZ
US008895596B2 (12) United States Patent (10) Patent No.: US 8,895,596 B2 Jiang et al. (45) Date of Patent: Nov. 25, 2014 (54) CYCLIC BENZMDAZOLE DERVATIVES (56) References Cited USEFUL AS ANT-DABETICAGENTS U.S. PATENT DOCUMENTS (75) Inventors: Jinlong Jiang, Scotch Plains, NJ (US); 5,596,025 A 1/1997 Oxman et al. Andrew J. Kassick, Scotch Plains, NJ 6,312,662 B1 11/2001 Erion et al. (US); Ahmet Kekec, Jersey City, NJ 6,489.476 B1 12/2002 Dang et al. (US); Iyassu K. Sebhat, Jersey City, NJ 7,098,220 B2 8, 2006 Rault et al. 7,268,145 B2 9, 2007 Matsumoto et al. (US) 2005, OO38068 A1 2/2005 Iyengar et al. 2005.0113283 A1 5/2005 Solow-Cordero et al. (73) Assignee: Merck Sharp & Dohme Corp, Rahway, 2005. O148643 A1 7/2005 Rui et al. NJ (US) 2005/O187277 A1 8/2005 Malti et al. 2005/0255415 A1 11/2005 Louwet et al. (*) Notice: Subject to any disclaimer, the term of this 2005/0272765 A1 12/2005 Feng et al. patent is extended or adjusted under 35 2006.0160872 A1 7/2006 Norman et al. 2006/0287356 Al 12/2006 Iyengaret al. U.S.C. 154(b) by 0 days. 2007, OO15665 A1 1/2007 Potluri et al. 2007/0O32529 A1 2/2007 Takagi et al. (21) Appl. No.: 13/578,302 2008. O1325O1 A1 6/2008 Sun et al. (22) PCT Fled: Feb. 21, 2011 FOREIGN PATENT DOCUMENTS (86) PCT NO.: PCT/US2011/025585 DE 33 16095 A1 11, 1983 EP O120403 A2 10, 1984 S371 (c)(1), EP O126030 A2 11, 1984 (2), (4) Date: Aug. -

Association of Oral Anticoagulants and Proton Pump Inhibitor Cotherapy with Hospitalization for Upper Gastrointestinal Tract Bleeding
Supplementary Online Content Ray WA, Chung CP, Murray KT, et al. Association of oral anticoagulants and proton pump inhibitor cotherapy with hospitalization for upper gastrointestinal tract bleeding. JAMA. doi:10.1001/jama.2018.17242 eAppendix. PPI Co-therapy and Anticoagulant-Related UGI Bleeds This supplementary material has been provided by the authors to give readers additional information about their work. Downloaded From: https://jamanetwork.com/ on 10/02/2021 Appendix: PPI Co-therapy and Anticoagulant-Related UGI Bleeds Table 1A Exclusions: end-stage renal disease Diagnosis or procedure code for dialysis or end-stage renal disease outside of the hospital 28521 – anemia in ckd 5855 – Stage V , ckd 5856 – end stage renal disease V451 – Renal dialysis status V560 – Extracorporeal dialysis V561 – fitting & adjustment of extracorporeal dialysis catheter 99673 – complications due to renal dialysis CPT-4 Procedure Codes 36825 arteriovenous fistula autogenous gr 36830 creation of arteriovenous fistula; 36831 thrombectomy, arteriovenous fistula without revision, autogenous or 36832 revision of an arteriovenous fistula, with or without thrombectomy, 36833 revision, arteriovenous fistula; with thrombectomy, autogenous or nonaut 36834 plastic repair of arteriovenous aneurysm (separate procedure) 36835 insertion of thomas shunt 36838 distal revascularization & interval ligation, upper extremity 36840 insertion mandril 36845 anastomosis mandril 36860 cannula declotting; 36861 cannula declotting; 36870 thrombectomy, percutaneous, arteriovenous -

Modifications to the Harmonized Tariff Schedule of the United States To
U.S. International Trade Commission COMMISSIONERS Shara L. Aranoff, Chairman Daniel R. Pearson, Vice Chairman Deanna Tanner Okun Charlotte R. Lane Irving A. Williamson Dean A. Pinkert Address all communications to Secretary to the Commission United States International Trade Commission Washington, DC 20436 U.S. International Trade Commission Washington, DC 20436 www.usitc.gov Modifications to the Harmonized Tariff Schedule of the United States to Implement the Dominican Republic- Central America-United States Free Trade Agreement With Respect to Costa Rica Publication 4038 December 2008 (This page is intentionally blank) Pursuant to the letter of request from the United States Trade Representative of December 18, 2008, set forth in the Appendix hereto, and pursuant to section 1207(a) of the Omnibus Trade and Competitiveness Act, the Commission is publishing the following modifications to the Harmonized Tariff Schedule of the United States (HTS) to implement the Dominican Republic- Central America-United States Free Trade Agreement, as approved in the Dominican Republic-Central America- United States Free Trade Agreement Implementation Act, with respect to Costa Rica. (This page is intentionally blank) Annex I Effective with respect to goods that are entered, or withdrawn from warehouse for consumption, on or after January 1, 2009, the Harmonized Tariff Schedule of the United States (HTS) is modified as provided herein, with bracketed matter included to assist in the understanding of proclaimed modifications. The following supersedes matter now in the HTS. (1). General note 4 is modified as follows: (a). by deleting from subdivision (a) the following country from the enumeration of independent beneficiary developing countries: Costa Rica (b). -

A Pharmaceutical Product for Hormone Replacement Therapy Comprising Tibolone Or a Derivative Thereof and Estradiol Or a Derivative Thereof
Europäisches Patentamt *EP001522306A1* (19) European Patent Office Office européen des brevets (11) EP 1 522 306 A1 (12) EUROPEAN PATENT APPLICATION (43) Date of publication: (51) Int Cl.7: A61K 31/567, A61K 31/565, 13.04.2005 Bulletin 2005/15 A61P 15/12 (21) Application number: 03103726.0 (22) Date of filing: 08.10.2003 (84) Designated Contracting States: • Perez, Francisco AT BE BG CH CY CZ DE DK EE ES FI FR GB GR 08970 Sant Joan Despi (Barcelona) (ES) HU IE IT LI LU MC NL PT RO SE SI SK TR • Banado M., Carlos Designated Extension States: 28033 Madrid (ES) AL LT LV MK (74) Representative: Markvardsen, Peter et al (71) Applicant: Liconsa, Liberacion Controlada de Markvardsen Patents, Sustancias Activas, S.A. Patent Department, 08028 Barcelona (ES) P.O. Box 114, Favrholmvaenget 40 (72) Inventors: 3400 Hilleroed (DK) • Palacios, Santiago 28001 Madrid (ES) (54) A pharmaceutical product for hormone replacement therapy comprising tibolone or a derivative thereof and estradiol or a derivative thereof (57) A pharmaceutical product comprising an effec- arate or sequential use in a method for hormone re- tive amount of tibolone or derivative thereof, an effective placement therapy or prevention of hypoestrogenism amount of estradiol or derivative thereof and a pharma- associated clinical symptoms in a human person, in par- ceutically acceptable carrier, wherein the product is pro- ticular wherein the human is a postmenopausal woman. vided as a combined preparation for simultaneous, sep- EP 1 522 306 A1 Printed by Jouve, 75001 PARIS (FR) 1 EP 1 522 306 A1 2 Description [0008] The review article of Journal of Steroid Bio- chemistry and Molecular Biology (2001), 76(1-5), FIELD OF THE INVENTION: 231-238 provides a review of some of these compara- tive studies. -

Pharmaceuticals As Environmental Contaminants
PharmaceuticalsPharmaceuticals asas EnvironmentalEnvironmental Contaminants:Contaminants: anan OverviewOverview ofof thethe ScienceScience Christian G. Daughton, Ph.D. Chief, Environmental Chemistry Branch Environmental Sciences Division National Exposure Research Laboratory Office of Research and Development Environmental Protection Agency Las Vegas, Nevada 89119 [email protected] Office of Research and Development National Exposure Research Laboratory, Environmental Sciences Division, Las Vegas, Nevada Why and how do drugs contaminate the environment? What might it all mean? How do we prevent it? Office of Research and Development National Exposure Research Laboratory, Environmental Sciences Division, Las Vegas, Nevada This talk presents only a cursory overview of some of the many science issues surrounding the topic of pharmaceuticals as environmental contaminants Office of Research and Development National Exposure Research Laboratory, Environmental Sciences Division, Las Vegas, Nevada A Clarification We sometimes loosely (but incorrectly) refer to drugs, medicines, medications, or pharmaceuticals as being the substances that contaminant the environment. The actual environmental contaminants, however, are the active pharmaceutical ingredients – APIs. These terms are all often used interchangeably Office of Research and Development National Exposure Research Laboratory, Environmental Sciences Division, Las Vegas, Nevada Office of Research and Development Available: http://www.epa.gov/nerlesd1/chemistry/pharma/image/drawing.pdfNational