Disease Discovery Classification of Lymphoid Neoplasms
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Recommendations on Scientific Collections As Research Infrastructures
wissenschaftsrat wr Drs. 10464-11 Berlin 28 January 2011 Recommendations on Scientific Collections as Research Infrastructures Contents Preamble 5 Summary 7 A. Scientific collections as research infrastructures 10 A.I Introduction 10 A.II Research based on scientific collections 11 A.III Definition of the subject matter 14 A.IV Definitions 15 A.V Aim of this statement 18 B. Critical analysis: status and function of scientific collections as research infrastructures 19 B.I Structural features 19 I.1 University collections 20 I.2 Non-university collections 23 B.II Resources 27 II.1 Finance 27 II.2 Accomodation 28 II.3 Human resources 29 B.III Use 30 III.1 Functions of scientific collections 30 III.2 Use for research 32 III.3 Intensity of use 33 B.IV Usability 33 IV.1 Management and quality assurance 34 IV.2 Care 35 IV.3 Access 35 IV.4 Documentation, indexing, digitisation 36 B.V Financial support options 39 B.VI Networking and coordination between institutions 41 C. Recommendations on the further development of scientific collections as research infrastructures 45 C.I Determining the status of a scientific collection 47 C.II Development of collection concepts 48 C.III Requirements for scientific collections as research infrastructure 50 III.1 Organisation and management 50 III.2 Resources 52 III.3 Indexing, accessibility, digitisation 53 C.IV Networking and organisation of scientific collections 55 C.V Financing and grants for scientific collections and collection-based research 57 Annexes 60 List of abbreviations 67 5 Preamble Scientific collections are a significant research infrastructure. -

LIST of OCCUPATIONAL DISEASES (Revised 2010)
LIST OF OCCUPATIONAL DISEASES (revised 2010) Identification and recognition of occupational diseases: Criteria for incorporating diseases in the ILO list of occupational diseases Occupational Safety and Health Series, No. 74 List of occupational diseases (revised 2010) Identification and recognition of occupational diseases: Criteria for incorporating diseases in the ILO list of occupational diseases INTERNATIONAL LABOUR OFFICE • GENEVA Copyright © International Labour Organization 2010 First published 2010 Publications of the International Labour Office enjoy copyright under Protocol 2 of the Universal Copyright Convention. Nevertheless, short excerpts from them may be reproduced without authorization, on condition that the source is indicated. For rights of reproduction or translation, application should be made to ILO Publications (Rights and Permissions), International Labour Office, CH-1211 Geneva 22, Switzerland, or by email: pubdroit@ ilo.org. The International Labour Office welcomes such applications. Libraries, institutions and other users registered with reproduction rights organizations may make copies in accordance with the licences issued to them for this purpose. Visit www.ifrro.org to find the reproduction rights organization in your country. ILO List of occupational diseases (revised 2010). Identification and recognition of occupational diseases: Criteria for incorporating diseases in the ILO list of occupational diseases Geneva, International Labour Office, 2010 (Occupational Safety and Health Series, No. 74) occupational disease / definition. 13.04.3 ISBN 978-92-2-123795-2 ISSN 0078-3129 Also available in French: Liste des maladies professionnelles (révisée en 2010): Identification et reconnaissance des maladies professionnelles: critères pour incorporer des maladies dans la liste des maladies professionnelles de l’OIT (ISBN 978-92-2-223795-1, ISSN 0250-412x), Geneva, 2010, and in Spanish: Lista de enfermedades profesionales (revisada en 2010). -

ICD-10 International Statistical Classification of Diseases and Related Health Problems
ICD-10 International Statistical Classification of Diseases and Related Health Problems 10th Revision Volume 2 Instruction manual 2010 Edition WHO Library Cataloguing-in-Publication Data International statistical classification of diseases and related health problems. - 10th revision, edition 2010. 3 v. Contents: v. 1. Tabular list – v. 2. Instruction manual – v. 3. Alphabetical index. 1.Diseases - classification. 2.Classification. 3.Manuals. I.World Health Organization. II.ICD-10. ISBN 978 92 4 154834 2 (NLM classification: WB 15) © World Health Organization 2011 All rights reserved. Publications of the World Health Organization are available on the WHO web site (www.who.int) or can be purchased from WHO Press, World Health Organization, 20 Avenue Appia, 1211 Geneva 27, Switzerland (tel.: +41 22 791 3264; fax: +41 22 791 4857; e-mail: [email protected]). Requests for permission to reproduce or translate WHO publications – whether for sale or for noncommercial distribution – should be addressed to WHO Press through the WHO web site (http://www.who.int/about/licensing/copyright_form). The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the World Health Organization concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. Dotted lines on maps represent approximate border lines for which there may not yet be full agreement. The mention of specific companies or of certain manufacturers’ products does not imply that they are endorsed or recommended by the World Health Organization in preference to others of a similar nature that are not mentioned. -

FAQ REGARDING DISEASE REPORTING in MONTANA | Rev
Disease Reporting in Montana: Frequently Asked Questions Title 50 Section 1-202 of the Montana Code Annotated (MCA) outlines the general powers and duties of the Montana Department of Public Health & Human Services (DPHHS). The three primary duties that serve as the foundation for disease reporting in Montana state that DPHHS shall: • Study conditions affecting the citizens of the state by making use of birth, death, and sickness records; • Make investigations, disseminate information, and make recommendations for control of diseases and improvement of public health to persons, groups, or the public; and • Adopt and enforce rules regarding the reporting and control of communicable diseases. In order to meet these obligations, DPHHS works closely with local health jurisdictions to collect and analyze disease reports. Although anyone may report a case of communicable disease, such reports are submitted primarily by health care providers and laboratories. The Administrative Rules of Montana (ARM), Title 37, Chapter 114, Communicable Disease Control, outline the rules for communicable disease control, including disease reporting. Communicable disease surveillance is defined as the ongoing collection, analysis, interpretation, and dissemination of disease data. Accurate and timely disease reporting is the foundation of an effective surveillance program, which is key to applying effective public health interventions to mitigate the impact of disease. What diseases are reportable? A list of reportable diseases is maintained in ARM 37.114.203. The list continues to evolve and is consistent with the Council of State and Territorial Epidemiologists (CSTE) list of Nationally Notifiable Diseases maintained by the Centers for Disease Control and Prevention (CDC). In addition to the named conditions on the list, any occurrence of a case/cases of communicable disease in the 20th edition of the Control of Communicable Diseases Manual with a frequency in excess of normal expectancy or any unusual incident of unexplained illness or death in a human or animal should be reported. -

Chronik Der Gesellschaft Für Pädiatrische Onkologie Und Hämatologie
Chronik der Gesellschaft für Pädiatrische Onkologie und Hämatologie U. Creutzig und J.-H. Klusmann für die GPOH auf Initiative von H. Jürgens Redaktionelle Mitarbeit: Britta Hildebrandt Herausgegeben von der GPOH und dem Kompetenznetz Pädiatrische Onkologie und Hämatologie Im Mai 2004 Prof. Dr. med. Ursula Creutzig Geschäftsführerin der GPOH und Leiterin der Koordinationszentrale Kompetenznetz Pädiatrische Onkologie und Hämatologie Universitäts-Kinderklinik Albert-Schweitzer-Str. 33 48129 Münster E-Mail: [email protected] Cand. med. Jan-Henning Klusmann Universität zu Lübeck E-Mail: [email protected] Die Chronik der GPOH Inhaltsverzeichnis Inhaltsverzeichnis I nhaltsverzeichnis . I V orwort . 1 E inführung . 2 B esonderheiten der Krebstherapie bei Kindern . 2 C hronik . 3 H istorische Daten zur Pädiatrischen Hämatologie . 3 H istorische Daten zur Pädiatrischen Onkologie . 3 E ntwicklung der Pädiatrischen Onkologie in den 60er Jahren . 4 Gründung der Deutschen Arbeitsgemeinschaft für Leukämie-Forschung und -Be- h andlung im Kindesalter e.V. (DAL) . 5 . 5 G ründung der Gesellschaft für Pädiatrische Onkologie (GPO) . 6 E ntwicklung der Pädiatrischen Hämatologie und Onkologie in Ostdeutschland . .. 6 S ituation in den 70er Jahren . 7 K inder-Tumorregister . 8 T herapieerfolge . 9 D eutsches Kinderkrebsregister . 1 0 E ntwicklung in den 80er und 90er Jahren . 1 2 Gründung der Gesellschaft für Pädiatrische Onkologie und Hämatologie (GPOH) . 1 2 A ktivitäten der DAL, GPO und GPOH bis heute ............... 1 2 P ädiatrische Hämatologie . 1 4 KOK – Konferenz Onkologischer Kranken- und Kinderkrankenpflege - eine A rbeitsgemeinschaft der Deutschen Krebsgesellschaft e.V. 1 4 T herapieoptimierungsstudien in der Pädiatrischen Onkologie . 1 6 A usgangslage . 1 6 Über blick über Therapieoptimierungsstudien der GPOH . 1 7 Arzneimittelrechtliche Probleme, Versuch der Lösung mit der Deutschen Krebs- ges ellschaft . -

15 Hereditary Renal Cancer
Hereditary Renal Cancer 239 15 Hereditary Renal Cancer Toshiyuki Miyazaki and Mutsumasa Takahashi CONTENTS 15.1 Introduction 15.1 Introduction 239 15.2 Histologic Subtypes of Renal Cancer 240 15.3 von Hippel-Lindau Disease 241 Renal cancer is diagnosed in over 30,000 Ameri- 15.3.1 Clinical Features 241 cans each year, accounting for approximately 15.3.2 Genetics 243 12,000 deaths annually. Smoking, obesity, and occu- 15.3.3 Imaging and Management 243 pational exposure have been implicated in the devel- 15.4 Hereditary Papillary Renal Cancer 248 opment of renal cancers but, in general, their cause 15.4.1 Clinical Features 248 Moyad 15.4.2 Genetics 248 remains obscure ( 2001). Although heredi- 15.4.3 Imaging and Management 249 tary renal cancer makes up only about 4% of the 15.5 Hereditary Leiomyoma Renal Cell Carcinoma 249 total number of cases, this percentage is expected 15.5.1 Clinical Features 249 to increase as a more complete understanding of 15.5.2 Genetics 250 the genetic causes of cancer is elucidated (Gago- 15.5.3 Imaging and Management 250 Dominguez 15.6 Birt-Hogg-Dubé Syndrome 251 et al. 2001). 15.6.1 Clinical Features 251 Increasing knowledge of hereditary renal cancer 15.6.2 Genetics 251 syndromes has provided insights into the mecha- 15.6.3 Imaging and Management 251 nisms of cancer development in the general popula- 15.7 Familial Renal Oncocytoma 252 tion and has assisted efforts to prevent and treat renal 15.7.1 Clinical Features 252 cancers. These cancers include von Hippel-Lindau 15.7.2 Genetics 252 15.7.3 Imaging and Management 252 disease, hereditary papillary renal cancer, hereditary 15.8 Medullary Carcinoma of Kidney 253 leiomyoma renal cell carcinoma, Birt-Hogg-Dubé 15.8.1 Clinical Features 253 syndrome, hereditary renal oncocytoma, familial 17.8.2 Genetics 253 renal oncocytoma, medullary carcinoma of kidney, 17.8.3 Imaging and Management 253 and tuberous sclerosis (Table 15.1; Choyke at al. -

History of the Statistical Classification of Diseases and Causes of Death
Copyright information All material appearing in this report is in the public domain and may be reproduced or copied without permission; citation as to source, however, is appreciated. Suggested citation Moriyama IM, Loy RM, Robb-Smith AHT. History of the statistical classification of diseases and causes of death. Rosenberg HM, Hoyert DL, eds. Hyattsville, MD: National Center for Health Statistics. 2011. Library of Congress Cataloging-in-Publication Data Moriyama, Iwao M. (Iwao Milton), 1909-2006, author. History of the statistical classification of diseases and causes of death / by Iwao M. Moriyama, Ph.D., Ruth M. Loy, MBE, A.H.T. Robb-Smith, M.D. ; edited and updated by Harry M. Rosenberg, Ph.D., Donna L. Hoyert, Ph.D. p. ; cm. -- (DHHS publication ; no. (PHS) 2011-1125) “March 2011.” Includes bibliographical references. ISBN-13: 978-0-8406-0644-0 ISBN-10: 0-8406-0644-3 1. International statistical classification of diseases and related health problems. 10th revision. 2. International statistical classification of diseases and related health problems. 11th revision. 3. Nosology--History. 4. Death- -Causes--Classification--History. I. Loy, Ruth M., author. II. Robb-Smith, A. H. T. (Alastair Hamish Tearloch), author. III. Rosenberg, Harry M. (Harry Michael), editor. IV. Hoyert, Donna L., editor. V. National Center for Health Statistics (U.S.) VI. Title. VII. Series: DHHS publication ; no. (PHS) 2011- 1125. [DNLM: 1. International classification of diseases. 2. Disease-- classification. 3. International Classification of Diseases--history. 4. Cause of Death. 5. History, 20th Century. WB 15] RB115.M72 2011 616.07’8012--dc22 2010044437 For sale by the U.S. -

Attachment D: Selection and Assignment of Oasis Diagnosis
ATTACHMENT D: SELECTION AND ASSIGNMENT OF OASIS DIAGNOSIS A. INTRODUCTION Attachment D was initially published to facilitate the introduction of V-code diagnosis reporting on the OASIS, (see OASIS-B1, 12/2002), effective October 1, 2003. The attachment was designed to clarify HHA implementation of the Official ICD-9-C M Coding Guidelines of 2002. The changes in OASIS diagnosis reporting in 2003 allowed HHAs the ability to assign V-codes to M0230 and M0240 and comply with the provisions of the Health Insurance Portability and Accountability Act (HIPAA, Title II). We are reissuing this document to promote accurate selection and assignment of the patient’s diagnosis on the current OASIS (OASIS B1 (1/2008). This document addresses the OASIS diagnoses items that pertain to the home health episode (i.e., M0230, M0240 and M0246) and will clarify CMS’ expectations specific to the assignment of V-codes to the OASIS as dictated by the revised ICD-9-C M coding guidelines effective October 2008. Additionally, with (HH PPS) refinements in January 2008, the HH PPS grouper was revised. If a V-code assigned to M0230/240 replaces a case mix diagnosis code, HHAs no longer must always code a numeric diagnosis code in the optional case mix diagnosis (M0246). ICD-9-C M coding guidelines state that certain rehabilitation and aftercare V-codes need a secondary diagnosis code in M0240 to describe a resolving condition or sequelae. If the diagnoses codes representing the underlying condition displaced by the V-code are case mix codes, the HH PPS grouper will look to M0240 to award appropriate points. -

Leopoldina News 03
Leopoldina news Deutsche Akademie der Naturforscher Leopoldina – Nationale Akademie der Wissenschaften Halle (Saale), 13 September 2012 03|2012 Dear members and friends of the Leopoldina, Leopoldina issues statement One of the biggest challenges facing society today is the energy supply of tomorrow. As on the opportunities and limits such, the German government’s work on ma- king the transition to of bioenergy renewable, sustainable energy sources affects cluded that bioenergy will not be able to us all. If, by 2050, make a quantitatively significant con- the majority of our tribution to Germany’s transition to re- energy is to come from newable energy sources. The statement renewable sources and points out that bioenergy requires more our carbon emissions surface area and is associated with high- are to fall by 80 percent, then we must see er greenhouse gas emissions than other the transition as a truly collaborative project renewable sources such as photovoltaic, that each and every one of us must support. solar thermal energy and wind energy. In Research is a crucial part of this project. addition, energy crops potentially com- The German National Academy of Sciences pete with food crops, the statement says. Leopoldina recently brought the issue of However, it also points out the areas energy into the public eye again when it where biogas, bioethanol and biodiesel published its statement „Bioenergy – Chances can be a climate-friendly alternative. For and Limits“. The document provides a nuanced example, the scientists recommend com- exploration of the potential of using bioenergy bining food production and bioenergy as an alternative energy source. -
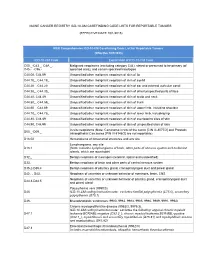
Casefinding Code List for Reportable Tumors (Effective 10/1/2015)
MAINE CANCER REGISTRY ICD-10-CM CASEFINDING CODE LISTS FOR REPORTABLE TUMORS (EFFECTIVE DATE 10/1/2015) MCR Comprehensive ICD-10-CM Casefinding Code List for Reportable Tumors (Effective 10/1/2015) ICD-10-CM Code Explanation of ICD-10-CM Code C00._-C43._, C4A._, Malignant neoplasms (excluding category C44), stated or presumed to be primary (of C45.-_-C96.-_ specified sites), and certain specified histologies C44.00, C44.09 Unspecified/other malignant neoplasm of skin of lip C44.10_, C44.19_ Unspecified/other malignant neoplasm of skin of eyelid C44.20_, C44.29_ Unspecified/other malignant neoplasm of skin of ear and external auricular canal C44.30_, C44.39_ Unspecified/other malignant neoplasm of skin of other/unspecified parts of face C44.40, C44.49 Unspecified/other malignant neoplasm of skin of scalp and neck C44.50_, C44.59_ Unspecified/other malignant neoplasm of skin of trunk C44.60_, C44.69_ Unspecified/other malignant neoplasm of skin of upper limb, including shoulder C44.70_, C44.79_ Unspecified/other malignant neoplasm of skin of lower limb, including hip C44.80, C44.89 Unspecified/other malignant neoplasm of skin of overlapping sites of skin C44.90, C44.99 Unspecified/other malignant neoplasm of skin of unspecified sites of skin In-situ neoplasms (Note: Carcinoma in situ of the cervix [CIN III-8077/2] and Prostatic D00._-D09._ Intraepithelial Carcinoma [PIN III-8148/2] are not reportable). D18.02 Hemangioma of intracranial structures and any site Lymphangioma, any site D18.1 (Note: Includes Lymphangioma of brain, other parts -

Endometriosis in Australia: Prevalence and Hospitalisations (Report)
Endometriosis in Australia: prevalence and hospitalisations Published August 2019 Around 1 in 9 women born in 1973–78 were diagnosed Endometriosis is a chronic condition that can be painful, with endometriosis by age 40–44 affect fertility and lead to reduced participation in school, work and sporting activities. The condition cost an estimated $7.4 billion in Australia in 2017–18, mostly through reduced quality of life and productivity losses (Ernst & Young 2019). This may be an underestimate, however, due to difficulties in diagnosing endometriosis There were around 34,200 and underdiagnosis. endometriosis-related hospitalisations in 2016–17 What is endometriosis? Endometriosis occurs when endometrial-like tissue, similar to the tissue normally found lining the uterus, is found in other parts of the body, such as the ovaries, fallopian tubes, peritoneum (the membrane lining the abdominal and pelvic cavities) and the outside of the uterus. These tissues are collectively known as endometriosis Nearly 4 in 5 (79%) and, like the endometrial tissue lining the uterus, they respond to endometriosis-related hormones released by the ovaries, causing bleeding. This leads hospitalisations in 2016–17 were among females to inflammation and scarring, which can cause painful ‘adhesions’ aged 15–44 joining together pelvic organs which are normally separate. The causes of endometriosis are unclear, but factors that seem to increase the risk of endometriosis include a family history of endometriosis and menstrual cycle factors such as early age at first period, short menstrual cycles, and heavy or long periods (Jean Hailes for Women’s Health 2017b). Around 15 of every Some people with endometriosis experience no symptoms; others 1,000 hospitalisations among may experience pain, heavy menstrual bleeding, bleeding between females aged 15–44 in 2016–17 periods, lethargy and reduced fertility, among other symptoms. -

Leopoldina News 01/2011 English
Leopoldina news Deutsche Akademie der Naturforscher Leopoldina – Nationale Akademie der Wissenschaften Halle (Saale), 2 March 2011 01/2011 Dear members Recommendations on and friends of the Leopoldina, Preimplantation genetic diagnosis (PGD) is a preimplantation genetic diagnosis topic that is currently being very strongly de- bated. Before summer recess, the Bundestag Ad-hoc statement by the Leopoldina favours allowing this diagnostic wants to decide the procedure subject to strict conditions nature of this legisla- tion. In advance of this decision there On 18 January the Leopoldina publi- is a great need for cally issued and published an ad-hoc information on the statement on preimplantation genetic complex, scientific, diagnosis (PGD). This was done in medical, ethical and conjunction with acatech – the German legal relationship. Academy of Science and Engineering, The Leopoldina has reacted in this matter the Berlin-Brandenburg Academy of and has produced a statement along with Science and Humanities (BBAW) and by other German academies. This paper has not the majority of the academies belonging only fostered discussion on the topic of PGD, to the Union of German Academies of but also triggered discussion about whether Science. The underlying message of the science academies should give advice of statement: PGD is to be placed on an policy and comment on ethical questions. equal footing with prenatal diagnosis This debate is important. As a National Aca- (PD) by lawmakers and access should demy of Science, the Leopoldina has taken be given in Germany under certain on a new duty. It has been commissioned conditions to those women affected. with advising on political and social matters This prevents having to terminate preg- based on scientific knowledge.