DETECTION of GELATIN in CULTURED BUTTERMILK and COTTAGE CHEESE by G
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

SOUR CREAM: Toward Semantic Processing of Recipes
SOUR CREAM: Toward Semantic Processing of Recipes Dan Tasse and Noah A. Smith CMU-LTI-08-005 Language Technologies Institute School of Computer Science Carnegie Mellon University 5000 Forbes Ave., Pittsburgh, PA 15213 www.lti.cs.cmu.edu SOUR CREAM: Toward Semantic Processing of Recipes Dan Tasse and Noah A. Smith School of Computer Science Carnegie Mellon University [email protected], [email protected] May 2008 1 Introduction We present preliminary work on SOUR CREAM (System to Organize and Understand Recipes, Capacitating Relatively Exciting Applications Meanwhile). The aim of this project is to develop new techniques for semantic parsing by focusing on the domain of cooking recipes. This report details the MILK meaning representation language and CURD, a database of recipes annotated in the MILK language. We also detail preliminary efforts at semantic processing using this dataset. 2 MILK: Minimal Instruction Language for the Kitchen In this section we present MILK, the Minimal Instruction Language for the Kitchen. In designing this language, we aimed to create a concise, yet complete, set of instructions that represent the actions demanded by imperative statements in recipes. MILK is based on first-order logic, but there is a notion of temporal order and creation/deletion of ingredients. It will provide a basic machine-readable target language for our parsing efforts; the MILK framework should allow a variety of useful applications. We have aimed for a medium-grained representation: a MILK translation of a recipe cannot identify in detail each action that occurs during cooking, but it will offer some useful information about each step in the recipe. -

Cream Ingredients
Cream Ingredients Cream is prepared from milk by centrifugal separation. United States standards require cream containing a minimum of 36% fat to be labeled “heavy whipping” cream. Cream used as an ingredient contains 36% to 40% fat. By standardizing with skim milk, cream of different fat levels can be produced. Light whipping cream and light (“coffee” or “table”) cream contain 30% to 36% and 18% to 30% fat, respectively. Specific homogenization and heat treatments bring about desirable grades of viscosity in cream products. Cream should be stored under refrigeration. It can be quick-frozen and stored frozen until used. Typical Composition for Fluid Milk Cream Products (%) Cream Product Water Fat Protein Lactose Ash Half-and-Half 80.2 11.5 3.1 4.5 0.7 Light Cream 74.0 18.3 2.9 4.2 0.6 Light Whipping Cream 62.9 30.5 2.5 3.6 0.5 Heavy Cream 57.3 36.8 2.2 3.2 0.5 Plastic Cream 18.2 80.0 0.7 1.0 0.1 Sour Cream, Cultured 71.0 21.0 3.2 4.3 0.7 Source: Chandan R. (1997), Dairy-Based Ingredients, Eagen Press, St. Paul, Minn. Cream Varieties Half-and-Half is a product containing between 10.5% and 18% milkfat, according to federal regulations. It can be pasteurized or ultrapasteurized and may be homogenized. The titratable acidity, expressed as lactic acid, is not less than 0.5%. If nutritive sweeteners or bulky flavoring ingredients are added, the final product must contain not less than 8.4% milkfat. -

Things You Need to Know About Sour Cream
Things You Need to Know About Sour Cream Many recipes call for sour cream and people ask what we think of it, so here’s “the skinny”. REGULAR SOUR CREAM is made from milk and here is a typical list of ingredients for example in the “all natural” brand Breakstone: cultured pasteurized grade a milk and cream, enzymes. 1 cup is approximately 480 calories. LOW FAT SOUR CREAM is made from low fat milk and here is a typical list of ingredients in the ”all natural” brand Breakstone: cultured pasteurized grade a milk and cream, contains less than 1% of agar, vitamin a palmitate, enzymes. 1 cup is approximately 240 calories. NON FAT SOUR CREAM is, well, not quite as “natural” as the higher fat cousins. Here are the ingredients in the same brand, Breakstone: cultured pasteurized grade a nonfat milk, dried corn syrup, food starch-modified, cream*, contains less than 2% of maltodextrin, artificial color, xanthan gum, natural flavor, vitamin a palmitate. *trivial source of fat. Notice all of the sweetener highlighted in yellow! How does sour cream compare to yogurt? Now, let’s compare the sour cream to nonfat Greek yogurt. FAT FREE PLAIN GREEK YOGURT is made from fat free milk and here are the ingredients in the Dannon brand: Cultured Grade A Nonfat Milk, Contains active yogurt cultures. 1 cup is approximately 120 calories. Yes, you can cook with it! Use plain Greek yogurt in any recipe that calls for sour cream! Fat Free Greek yogurt looks and tastes like sour cream, has fewer calories, no fat and no sweeteners! Try it out when cooking! Be sure you are getting PLAIN greek yogurt. -

THE QUARANTINE MENU Appetizers Wings Soups Salads
THE QUARANTINE MENU Appetizers Veggie Platter 6.99 Inside Out Skins 6.99 Tzatziki & Hummus served with carrots, A baked potato flipped inside out breaded with cucumbers and grilled pita bread panko and deep fried. Topped with cheese, bacon, green onions, and sour cream Artichoke & Spinach Dip 8.99 House made with spinach, a blend of 6 cheeses Stuffed Mushrooms 6.99 & artichokes served with tortilla chips Sausage, cream cheese and spices (subject to availability) Chicken Tenders 8.99 Breaded boneless chicken strips plain or tossed in Nachos 7.99 choice of sauce. See Wing Sauce List below. Platter of tortilla chips topped with melted cheddar, onion, tomato, and jalapenos Bavarian Soft Pretzel Sticks 6.99 Add Beef or Chicken 2.00 Served with your choice of our signature beer Add Sour Cream 0.75 cheese or nacho cheese Wings 6 wings 6.99 12 wings 12.99 20 wings 20.99 Hand breaded and deep fried to perfection. Tossed in your choice of sauce Sauce: Hot, Mild, Parmesan Garlic, Thai Chili, Honey BBQ or BBQ Soups French Onion 5.99 Classic Tomato (subject to availability) 4.59 Salads (Add chicken $3.00 Add salmon $5.00) Chopped Salad 11.99 House Salad 5.99 Chopped salad greens, cucumbers, red onion, Mixed greens, cucumbers, tomatoes & red onions artichokes, green onions, bacon, & cherry served with your choice of dressing tomatoes tossed with ranch dressing Taco Salad 7.99 Cobb Salad 11.99 Mixed greens, seasoned beef, green onion, Chopped salad greens, cherry tomatoes, ham, red tomato, and cheese. Served in a Tortilla onion, sliced avocado, smoked turkey, blue cheese shell (Add Sour Cream 0.75) & bacon served with your choice of dressing Salad Dressings: Parmesan Peppercorn Greek Salad 10.99 (House), Ranch, Caesar, Tomato Vinaigrette, Fresh greens, cucumber, feta cheese, red onion, Honey Mustard, Red French, oil & vinegar, and black olives served with your choice of Thousand Island, and Italian. -

Bar Appetizers
--- Bar Appetizers --- Arnie’s Guacamole $9.00 Crab Salad Guacamole $14.00 Lime Juice, Cilantro, Tomatoes, Fresh Jumbo Lump Claw Meat, Jalapeño, Onion Charred Spring Onions Bacon Guacamole $10.00 Mango Guacamole $10.00 House Smoked Bacon, Roasted Corn Salsa, Grilled Mango, Pickled Fresno Chili, Queso Fresco Smoked Paprika Guacamole Sampler $15.00 Pick any three of our fresh made Guacamoles Wood Baked Queso $10.00 A blend of Mexican Cheeses, House-made Chorizo, Charred Peppers & Onions, Topped with Cilantro & Queso Fresco Chorizo Nachos $10.00 Pepperjack Sauce, Cilantro, Lime Pickled Onion, Chipotle Salsa Chips & Salsa $5.00 Roasted & Fresh Salsa with Warm Tortilla Chips --- Arnie’s Desserts --- Chipotle Chocolate Skillet Brownie $8.00 Honey Lime Caramel & Vanilla Bean Ice Cream Tequila Lime Cheesecake $8.00 Whipped Cream Traditional Flan $8.00 Fresh Berry Compote Arnie’s Barn is a 150 year old barn found right in Arnold Palmer’s own back yard in Latrobe, PA. Johnny Morris commissioned local Ozark craftsman Danny Schwartz and his family members to carefully disassemble the barn and its 46-foot timbers, numbering each piece, transporting and re-erecting the barn at Top of the Rock to serve as the golf pro shop and a second restaurant. Some of the timbers from the barn – which included the now extinct American chestnut – are so old, Top of the Rock historians are carbon dating them. When the barn was built 150 years ago, some of those timbers were already well over 100 years old. From Arnie’s Barn, guests enjoy magnificent views of the practice facility along with unique memorabilia and golf gear found nowhere else. -
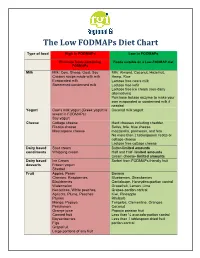
The Low Fodmaps Diet Chart
The Low FODMAPs Diet Chart Type of food High in FODMAPs Low in FODMAPs Eliminate foods containing Foods suitable on a Low-FODMAP diet FODMAPs Milk Milk: Cow, Sheep, Goat, Soy Milk: Almond, Coconut, Hazelnut, Creamy soups made with milk Hemp, Rice Evaporated milk Lactose free cow’s milk Sweetened condensed milk Lactose free kefir Lactose free ice cream (non-dairy alternatives) Purchase lactase enzyme to make your own evaporated or condensed milk if needed Yogurt Cow’s milk yogurt (Greek yogurt is Coconut milk yogurt lowest in FODMAPs) Soy yogurt Cheese Cottage cheese Hard cheeses including cheddar, Ricotta cheese Swiss, brie, blue cheese, Mascarpone cheese mozzarella, parmesan, and feta No more than 2 tablespoons ricotta or cottage cheese Lactose free cottage cheese Dairy based Sour cream Butter-limited amounts condiments Whipping cream Half and Half- limited amounts Cream cheese- limited amounts Dairy based Ice Cream Sorbet from FODMAPs friendly fruit desserts Frozen yogurt Sherbet Fruit Apples, Pears Banana Cherries, Raspberries, Blueberries, Strawberries Blackberries Cantaloupe, Honeydew-portion control Watermelon Grapefruit, Lemon, Lime Nectarines, White peaches, Grapes-portion control Apricots, Plums, Peaches Kiwi, Pineapple Prunes Rhubarb Mango, Papaya Tangelos, Clementine, Oranges Persimmon Coconut Orange juice Papaya passion fruit Canned fruit Less than ¼ avocado-portion control Boysenberries Less than 1 tablespoon dried fruit Figs portion control Grapefruit Large portions of any fruit Limit consumption to one low FODMAPs fruit -

Catering Menu
B R EA K FA ST BREAKFAS T BUFFET Basic Breakfast 4.50 per person FOR PARTIES OF 10 OR MORE Selection of Freshly Baked Muffins and Bagels / Breakfast Buffet 12 per person Cream Cheese / Butter / Assorted Jellies Scrambled Eggs / Roasted Potatoes / Pork Sausage / Bacon / Bagels / Business Breakfast 6.50 per person Cream Cheese / Butter / Assorted Jellies Selection of Freshly Baked Muffins and Bagels / Cream Cheese / Butter / Assorted Jellies / Fruit Tray Executive Breakfast 9 per person A L A C A RT E Selection of Freshly Baked Muffins and Bagels / Yogurt Parfait 5 each Cream Cheese / Butter / Assorted Jellies / Orange Juice 2.50 each Fruit Tray / Orange Juice CATERING Fruit Tray Small (For 15 people) 40/tray VIP Breakfast 12 per person Large (For 30 people) 60/tray MENU Selection of Freshly Baked Muffins and Bagels / 16 oz Soda Pepsi, Diet Pepsi, Sierra Mist 2.25 each Cream Cheese / Butter / Assorted Jellies / 16 oz Water 2.25 each Fruit Tray / Orange Juice / Starbucks Coffee Cookies 2.25 each (Decaf or Regular) PLACE ORDERS Hummus Platter With Veggies and Pita BY 2 PM Create A Breakfast Sandwich 5 per person (For 10-15 people) 45/tray THE PREVIOUS DAY Egg / Cheese / Choice of Bacon, Sausage or Canadian Bacon / Choice of English Muffin or Toast COFFEE OPEN 7 AM TO 4 PM ORDERING & DELIVERY We proudly brew 30 South Wacker Drive Chicago, Illinois 60606 To order call 312-559-1835. Includes Creamers / Sugar / 312-559-1835 Orders must be place by 2 pm the previous day. Stir Stix / Napkins For parties of 8 or more 3 per person BOX LUNCHES 14 EACH ORD ER BY 2 PM PREVIOUS DAY HOT BUFFETS DELI SANDWICHES & WR APS MARKET FRESH SALADS FOR PARTIES OF 10 OR MORE Served with choice of Chips, Fruit or Mixed Green Salad, Served with Fresh Bread, Butter, Chips and Cookie Taco Bar 15 per person and Cookie. -

Salads Starters Soups Entrees
Home of the Perfect Pint Soups Salads Cup $6 Bowl $8 Snug Caesar Salad Romaine lettuce, parmesan cheese, croutons Homemade Chili tossed with Caesar dressing $12 Top with cheese & onion add .50 Snug House Salad Homemade New England Clam Chowder A blend of Romaine & iceberg lettuce Thick and creamy, with cucumbers, tomatoes, Bermuda onions, served with oyster crackers carrots, red & green peppers $12 Greek Salad Romaine lettuce, feta cheese, banana peppers, Starters red & green peppers, Bermuda onions, Pub Pretzels cucumbers & black olives $12 Crispy, salty pretzels with Include your favorite to any salad above: homemade Guinness mustard sauce $9 Grilled chicken breast or Snug turkey tips add $8 Mozzarella Sticks Snug sirloin tips, grilled salmon filet or gulf shrimp add $10 With marinara dipping sauce $10 Snug Cobb Salad Country Style Potato Skins Grilled chicken breast, diced tomato, crispy bacon, Topped with Monterey Jack cheese & crispy bacon avocado, hardboiled egg, bleu cheese over a bed of served with sour cream $10 blended Romaine & iceberg lettuce $16 Basket of Crispy Snug Wings Always fresh, never frozen golden fried chicken wings in your choice of plain, teriyaki, sweet chili or Buffalo Entrees served with carrots, celery sticks & bleu cheese dressing $13 Our Award Winning “Snug Steak Tips” Basket of Chicken Fingers Everyone’s favorite char-grilled sirloin tips Always fresh never frozen, hand battered chicken tenders, marinated in our secret recipe $24 served with crispy French fries in your choice Traditional Fish ‘n Chips of -

Superbrands Finland 36 Market Ingman Foods Has Been
product Ingman offers consumers a wide and versatile range of liquid milk prod- ucts, chilled dairy products, ice creams and cheese, to cover daily nutritional Annually the Group processes about needs from breakfast to evening 300 million liters of milk into liquid milk and meals. New products are launched chilled dairy products, ice-cream, cheese and frequently to meet the require- butter. Innovative product development has ments of a modern consumer. always been a major resource at Ingman Foods. The Group was the first to introduce recent developments many new products to the Finnish market, The food industry has become more inter- e.g. curdled milk, natural unflavored yogurt, national and the fast changing competitive and yogurt with fruit, layered yogurt, tradi- situation brings harder challenges also for tional and flavored cottage cheese, créme fra- the ice cream market. Ingman’s message to iche, smetana sour cream, curd, juice, packaged Finnish consumers is that it remains a strongly and giant ice cream cones, premium ice cream domestic ice cream producer. and functional products such as ice cream, tion for emphasizing domestic values and its The product mix in all segments has been flavored sour milk, cottage cheese and 100% commitment to Finnish raw materials. Ingman systemically developed to meet the require- unsweetened juice. Ingman Foods was also has also been promoting the ‘Food from ments of the market. The demand has been the pioneering Finnish manufacturer of soya- Finland’ campaign actively, and it has kept the responded by creating a large selection of based ice cream, yogurt and pudding. consumers well informed. -

Enchiladas Y Burritos Botanas Tacos Ensaladas
Mexican Restaurant & Tequila Bar Botanas Tacos Appetizers Skirt Steak 15.99 Guacamole 9.99 Grilled marinated skirt steak, three corn or flour tortillas, lettuce, tomato, rice and Hass Avocado, cilantro, onion, tomato, add Chihuahua Cheese 1.00 lime, jalapeńo beans Traditional Queso Fundido 8.99 Al Pastor 14.99 Chorizo, pico de gallo,Chihuahua cheese Three corn or flour tortillas, marinated pork, w/corn or flour tortillas cilantro, onion, pineapple, rice and beans Pescado 14.99 White Queso Dip 4oz. 2.99 10oz. 7.99 Three corn or flour tortillas, grilled White queso with a blend of mahi mahi with verde sauce or fried mahi Mexican peppers and seasoning mahi with chipotle mayo, shredded cabbage, black beans and elote (corn on the cob) Fiesta Nachos 9.99 Refried beans, Chihuahua cheese, tomato, Picadillo 13.99 jalapeńo, guacamole, sour cream Three corn or flour tortillas, ground beef, add Ground Beef or Chicken Tinga 2.99 lettuce, tomato, cheese rice and beans Quesadilla 9.99 Chicken Tinga 14.99 Chihuahua cheese, sour cream, guacamole Three corn or flour tortillas, Chicken braised add Chicken Tinga 1.99; Vegetable Mix 1.99; Skirt Steak 3.99 with tomato and chipotle, lettuce, tomatoes, Chihuahua cheese, rice, refried beans Casa Bonita Quesadilla 14.99 Skirt steak, Chihuahua cheese, onion, tomato, Carnitas 13.99 cilantro, jalapeńo, guacamole, sour cream Three corn or flour tortillas, pork slowly roasted golden brown, cilantro, onion, Ceviche 9.99 rice and beans Tilapia, onion, carrot, tomato, cucumber, orange, lime. Served with Red cabbage Barbacoa 14.99 Three corn or flour tortillas, beef slowly Tacos Dorados 14.99 braised with chilies, cilantro, onion, Four deep fried corn tortillas with your rice and beans choice of protein (ground beef, chicken Mushroom and Ancho Chile 14.99 tinga, carnitas), smothered in a creamy guacamole sauce. -

Nature's Best Dairy Sour Cream Flyer
Sour Cream Simply Delicious! WELCOME TO NATURE’S BEST DAIRY® Exclusively from Performance Foodservice the Nature’s Best Dairy® brand brings you the best in high quality dairy products, from milk and butter, heavy creams and cottage cheese to eggs and ice cream all sourced from across the land. Nature’s Best Dairy® combines our nation’s rich dairy farming heritage with the promise of excellence in food quality, environmental sustainability, product integrity, safety and social responsibility. A cold glass of milk, a scoop of ice cream, butter on a bagel—deliver a simple pleasure that’s simply more delicious with Nature’s Best Dairy®! SOUR CREAM TOPS EVERYTHING We proudly present Nature’s Best Dairy® Sour Cream. Nature’s Best Dairy® Sour Cream is made from high quality milk and live cultures to give an extra-tangy zip to any recipe. Nature’s Best Dairy® Sour Cream is a great base for various dips and dressings, and as a traditional topping for baked potatoes, nachos and desserts. It is also a great ingredient to add to cakes, cookies, donuts and scones. Whether you need a cool, tart tang or a mild note to mellow a spicy dish, Nature’s Best Dairy® Sour Cream will give your recipes with right flavor. Simply Delicious! Sour Cream FEATURES AND BENEFITS PRODUCTS • Formulated with Grade A pasteurized cream Item # Description Pack Size to maintain body and silky consistency. 316321 Hvy Sour Cream 1 32 LB • Holds up in hot applications and resists 201096 Hvy Sour Cream 2 5 LB separation for optimal plate presentation. -
Creamy Guacamole Burgers Blueapron.Com with Elote-Style Corn on the Cob
Creamy Guacamole Burgers blueapron.com with Elote-Style Corn on the Cob 4 SERVINGS | 20–30 MINS Mexican-style flavors abound in these burgers thanks to a cooling sauce of sour Serve a bottle of Blue Apron wine with this symbol: Crisp & Minerally. cream mixed with guacamole that tops beef patties elevated with classic spices blueapron.com/wine like paprika, ancho chile powder, and more. Ingredients 1 1/8 lbs Ground Beef 1/4 cup Mayonnaise 4 Potato Buns 1/4 cup Sour Cream 4 ears of Corn 1 Tbsp Mexican Spice Blend* 1 Lime 1/2 cup Guacamole 2 Tbsps Grated Cotija Cheese *Ancho Chile Powder, Smoked Paprika, Garlic Powder, Ground Cumin & Dried Mexican Oregano 1 Prepare the ingredients 4 Make the lime mayo & dress the corn • Fill a large pot 3/4 of the way • Meanwhile, in a bowl, up with salted water; cover combine the mayonnaise, and heat to boiling on high. remaining spice blend, and • Wash and dry the fresh the juice of the remaining produce. lime half; season with salt and pepper. • Remove the husks and silks from the corn. • When cool enough to handle, evenly spread the • Halve the buns. lime mayo onto the cooked • Halve the lime crosswise. corn. Top with the cheese. • In a bowl, combine the sour cream, guacamole, and the juice of 1 lime half; season with salt and pepper. Stir to combine. 5 Toast the buns & serve your dish • Working in batches if 2 Form & cook the patties necessary, add the halved • In a large bowl, combine buns, cut side down, to the beef and all but a pinch the pan of reserved fond.