Luminescence Based Detection of Trinitrophenol and Aromatic Organophosphorous Pesticides Using a Coordination Polymer
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Teratogenicity and Embryotoxicity of Organophosphorus Compounds in Animal Models - a Short Review
Mil. Med. Sci. Lett. (Voj. Zdrav. Listy) 2012, vol. 81(1), p. 16-26 ISSN 0372-7025 DOI: 10.31482/mmsl.2012.003 REVIEW ARTICLE TERATOGENICITY AND EMBRYOTOXICITY OF ORGANOPHOSPHORUS COMPOUNDS IN ANIMAL MODELS - A SHORT REVIEW Syed M Nurulain and M Shafiullah Department of Pharmacology and Therapeutic, Faculty of Medicine and Health Sciences, UAE University, AlAin, UAE. P.O.Box 17666, Alain, UAE Received 9 th December 2011. Revised 12 th February 2012. Published 9 th March 2012. Summary Organophosphorus compounds (OPCs) are a wide group of compounds both structurally and functionally. Each OPC has a unique toxicological profile. The exposure to this type of poison is not limited only to certain occupationally exposed people but also to children, women, pregnant women; all have chances to be exposed to this poison. During the recent past years it has been reported in many poison epidemiological studies and case reports that exposure of OPCs during pregnancy caused malformed fetuses, neural tube defect (NTD) and shortening of pregnancy. The literature for animal models reveals inconclusive evidence. The generalized view is that they are neither teratogenic nor embryotoxic. But it is not true. There is a lack of systematic study and scarcity of reports on the topic. The present study was undertaken to investigate the teratogenicity induced by organophosphorus compounds in different animal models by literature review. Literature was searched by Toxicology Data NetWork (TOXNET), Developmental and Reproductive Toxicology Database (DART), Toxicology Literature Online (TOXLINE), Hazardous Substances Data Bank (HSDB), Pubmed Central, Entrez-Pubmed, Science Direct, Directory Of Open Access Journal (DOAJ), Google Scholar and International Program on Chemical Safety (IPCS-INCHEM), Embase. -

Triazofos 60% LC
Excel Crop Care Limited, India Material Safety Data Sheet Triazofos 60% LC 1. Chemical Product and Company Identification Product Name : Triazofos 60% LC Formula : C12 H16N3O3PS Molecuar Weight : 313.3 : O,O-diethyl O-(1-phenyl-1H-1,2,4-triazol-3-yl) phosphorothioate. Chemical Name Manufacturer : Excel Crop Care Limited 184 / 87, S. V. Road, Jogeshwari (West), Mumbai, (India). Pin code: 400 102 Contact no: +912266464200 Emergency Contact Details : +91 22 42522201 : +91 22 42522202 : +91 278 2212320 2. Composition/Information on Ingredients Component CAS No. % W/W content Triazofos 24017-47-8 60 minimum Xylene 1330-20-7 Q. S. 3. Hazard Identification Emergency Overview Appearance and odour : Pale yellow to dark brown clear liquid, with typical phosphate ester odour Warning Statements : Keep out of reach of children Potential Health Effects Likely Routs of Exposure : Skin contact, Inhalation and Ingestion Eye Contact :Unlikely to cause irritation to eye Skin contact : Not irritating to skin Ingestion :If swallowed, this substance is considered to be toxic and likely to Cause damages to internal organ Version: ECCL/MSDS/Triazo60LC/1 © Excel Crop Care Ltd Page 1 of 7 DoC: Sept 14, 2014 Private Document DoU: Sept 25, 2014 Issue Date: Oct 1, 2014 Excel Crop Care Limited, India Material Safety Data Sheet Triazofos 60% LC Inhalation : Repeated exposure may cause cholinesterase inhibition. Environmental Hazards : The product is an organophosphate insecticide 4. First aid measures and Antidote Emergency and First Aid Procedure – If in Eyes : Immediately flush with plenty of clean water for 15-20 minutes. Remove contact lenses if present after 5 minutes of washing. -

The List of Extremely Hazardous Substances)
APPENDIX A (THE LIST OF EXTREMELY HAZARDOUS SUBSTANCES) THRESHOLD REPORTABLE INVENTORY RELEASE QUANTITY QUANTITY CAS NUMBER CHEMICAL NAME (POUNDS) (POUNDS) 75-86-5 ACETONE CYANOHYDRIN 500 10 1752-30-3 ACETONE THIOSEMICARBAZIDE 500/500 1,000 107-02-8 ACROLEIN 500 1 79-06-1 ACRYLAMIDE 500/500 5,000 107-13-1 ACRYLONITRILE 500 100 814-68-6 ACRYLYL CHLORIDE 100 100 111-69-3 ADIPONITRILE 500 1,000 116-06-3 ALDICARB 100/500 1 309-00-2 ALDRIN 500/500 1 107-18-6 ALLYL ALCOHOL 500 100 107-11-9 ALLYLAMINE 500 500 20859-73-8 ALUMINUM PHOSPHIDE 500 100 54-62-6 AMINOPTERIN 500/500 500 78-53-5 AMITON 500 500 3734-97-2 AMITON OXALATE 100/500 100 7664-41-7 AMMONIA 500 100 300-62-9 AMPHETAMINE 500 1,000 62-53-3 ANILINE 500 5,000 88-05-1 ANILINE,2,4,6-TRIMETHYL- 500 500 7783-70-2 ANTIMONY PENTAFLUORIDE 500 500 1397-94-0 ANTIMYCIN A 500/500 1,000 86-88-4 ANTU 500/500 100 1303-28-2 ARSENIC PENTOXIDE 100/500 1 THRESHOLD REPORTABLE INVENTORY RELEASE QUANTITY QUANTITY CAS NUMBER CHEMICAL NAME (POUNDS) (POUNDS) 1327-53-3 ARSENOUS OXIDE 100/500 1 7784-34-1 ARSENOUS TRICHLORIDE 500 1 7784-42-1 ARSINE 100 100 2642-71-9 AZINPHOS-ETHYL 100/500 100 86-50-0 AZINPHOS-METHYL 10/500 1 98-87-3 BENZAL CHLORIDE 500 5,000 98-16-8 BENZENAMINE, 3-(TRIFLUOROMETHYL)- 500 500 100-14-1 BENZENE, 1-(CHLOROMETHYL)-4-NITRO- 500/500 500 98-05-5 BENZENEARSONIC ACID 10/500 10 3615-21-2 BENZIMIDAZOLE, 4,5-DICHLORO-2-(TRI- 500/500 500 FLUOROMETHYL)- 98-07-7 BENZOTRICHLORIDE 100 10 100-44-7 BENZYL CHLORIDE 500 100 140-29-4 BENZYL CYANIDE 500 500 15271-41-7 BICYCLO[2.2.1]HEPTANE-2-CARBONITRILE,5- -

Application of Dried Blood Spots for the Determination of Organophosphorus Pesticides by GC-MS/MS
UNIVERSIDADE DA BEIRA INTERIOR Ciências Application of dried blood spots for the determination of organophosphorus pesticides by GC-MS/MS Sofia Pires Seixo Soares Dissertação para obtenção do Grau de Mestre em Bioquímica (2º ciclo de estudos) Orientador: Doutor Mário Jorge Dinis Barroso Co-orientador: Professora Doutora Maria Eugenia Gallardo Alba Covilhã, junho de 2018 Agradecimentos A realização de uma dissertação de mestrado é o culminar de muitas horas de estudo, reflexão e trabalho pessoal, mas representa também uma etapa que não seria possível sem o contributo fundamental de várias pessoas. À Doutora Eugénia Gallardo e ao Doutor Mário Barroso, como excelentes mentores que são, quero agradecer toda a orientação, motivação, incentivo e confiança. Toda a ajuda e partilha de experiência e conhecimento durante todo este percurso. Ao Tiago Rosado, pela imprescindível ajuda e disponibilidade constante, bem como pela amizade. À Joana Gonçalves e ao Ângelo Luís um agradecimento especial pela amizade e carinho demonstrado ao longo deste ano. A todos, quero agradecer pelo bom ambiente proporcionado. Aos meus pais Ana Soares e José Soares, a quem dedico este feito, por me darem a possibilidade de concretizar conquistas como esta, pelo apoio incondicional, por sempre terem acreditado em mim e por fazerem parte deste momento. Aos meus avós e restante família, por todo o apoio e preocupação. Um agradecimento particular à Inês Ramos, à Rita Marques, à Maria Cunha e ao Pedro Batista pelo companheirismo, amizade e apoio de há tantos anos. E por último, mas nunca menos importante, um enorme obrigada ao meu namorado António Fernandes por toda a paciência, pelo incansável apoio, carinho e ajuda. -

Redalyc.Luminescence Based Detection of Trinitrophenol And
Journal of the Mexican Chemical Society ISSN: 1870-249X [email protected] Sociedad Química de México México Kaur, Rupinder; Chhibber, Manmohan; Mahata, Partha; Mittal, Susheel K. Luminescence Based Detection of Trinitrophenol and Aromatic Organophosphorous Pesticides Using a Coordination Polymer Journal of the Mexican Chemical Society, vol. 61, núm. 4, 2017, pp. 336-341 Sociedad Química de México Distrito Federal, México Available in: http://www.redalyc.org/articulo.oa?id=47554335008 How to cite Complete issue Scientific Information System More information about this article Network of Scientific Journals from Latin America, the Caribbean, Spain and Portugal Journal's homepage in redalyc.org Non-profit academic project, developed under the open access initiative J. Mex. Chem. Soc. 2017, 61(4),336-341 Article © 2017, Sociedad Química de México ISSN 1870-249X Luminescence Based Detection of Trinitrophenol and Aromatic Organophosphorous Pesticides Using a Coordination Polymer Rupinder Kaur,1 Manmohan Chhibber,1 Partha Mahata2 and Susheel K. Mittal*1 1 School of Chemistry & Biochemistry, Thapar University, Patiala-147004, India. Email: [email protected] 2 Department of Chemistry, Jadavpur University, Kolkata-700 032, India Email: [email protected] Received February 3rd, 2017; Accepted September 18th, 2017 Abstract: The fluorescent properties of a coordination polymer Resumen: Las propiedades fluorescentes del polímero de coordinación (CP), 1, were used as turn-on and turn-off detector for nitroaromatics (CP), 1, fueron utilizadas -

Investigation on the Behavior of Pesticides in Atmosphere
Aerosol and Air Quality Research, 11: 783–790, 2011 Copyright © Taiwan Association for Aerosol Research ISSN: 1680-8584 print / 2071-1409 online doi: 10.4209/aaqr.2010.10.0085 Investigation on the Behavior of Pesticides in Atmosphere Pasquale Avino1, Giuseppe Cinelli2, Ivan Notardonato2, Mario V. Russo2* 1 DIPIA, INAIL (ex-ISPESL), via Urbana 167, 00184 Rome, Italy 2 Faculty of Agriculture, University of Molise, via De Sanctis, Campobasso, Italy ABSTRACT Although pesticides are widely used in agriculture, they and in particular the relative residues in foodstuffs, water and atmosphere, may cause remarkable sanitary problems due to the harmful effects (carcinogenic and mutagenic effects) on the human health. In fact, their spread in waters and atmosphere can produce undesired effects on various organisms and/or water contamination. This paper shows an analytical approach based on XAD-2 adsorbent and GC analysis for evaluating the pesticide trend in atmosphere: in particular, the pesticides investigated are omethoate, dicrotofos, disulfoton, dimethoate, parathion methyl, formothion, paraoxon ethyl, malaoxon, parathion ethyl, iodofenfos and triazofos. For the analytical methodology a linearity response was obtained (r2 = 0.9988) in GC-NPD whereas the limits of detection range between 2 and 5 pg/μL in GC-NPD with a Relative Standard Deviation below 9.5. Finally, this approach has been successfully applied to real samples: the results show that dimethoate concentration decreases with increasing distance from the sampling site but it is still persistent in atmosphere after few days from the pesticide spraying. Keywords: Pesticides; XAD-2 adsorbent; GC analysis; Atmosphere; Air quality. INTRODUCTION 2009). One of the most important OP reactions is water hydrolysis. -
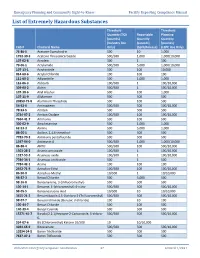
List of Extremely Hazardous Substances
Emergency Planning and Community Right-to-Know Facility Reporting Compliance Manual List of Extremely Hazardous Substances Threshold Threshold Quantity (TQ) Reportable Planning (pounds) Quantity Quantity (Industry Use (pounds) (pounds) CAS # Chemical Name Only) (Spill/Release) (LEPC Use Only) 75-86-5 Acetone Cyanohydrin 500 10 1,000 1752-30-3 Acetone Thiosemicarbazide 500/500 1,000 1,000/10,000 107-02-8 Acrolein 500 1 500 79-06-1 Acrylamide 500/500 5,000 1,000/10,000 107-13-1 Acrylonitrile 500 100 10,000 814-68-6 Acrylyl Chloride 100 100 100 111-69-3 Adiponitrile 500 1,000 1,000 116-06-3 Aldicarb 100/500 1 100/10,000 309-00-2 Aldrin 500/500 1 500/10,000 107-18-6 Allyl Alcohol 500 100 1,000 107-11-9 Allylamine 500 500 500 20859-73-8 Aluminum Phosphide 500 100 500 54-62-6 Aminopterin 500/500 500 500/10,000 78-53-5 Amiton 500 500 500 3734-97-2 Amiton Oxalate 100/500 100 100/10,000 7664-41-7 Ammonia 500 100 500 300-62-9 Amphetamine 500 1,000 1,000 62-53-3 Aniline 500 5,000 1,000 88-05-1 Aniline, 2,4,6-trimethyl- 500 500 500 7783-70-2 Antimony pentafluoride 500 500 500 1397-94-0 Antimycin A 500/500 1,000 1,000/10,000 86-88-4 ANTU 500/500 100 500/10,000 1303-28-2 Arsenic pentoxide 100/500 1 100/10,000 1327-53-3 Arsenous oxide 100/500 1 100/10,000 7784-34-1 Arsenous trichloride 500 1 500 7784-42-1 Arsine 100 100 100 2642-71-9 Azinphos-Ethyl 100/500 100 100/10,000 86-50-0 Azinphos-Methyl 10/500 1 10/10,000 98-87-3 Benzal Chloride 500 5,000 500 98-16-8 Benzenamine, 3-(trifluoromethyl)- 500 500 500 100-14-1 Benzene, 1-(chloromethyl)-4-nitro- 500/500 -

List of Lists
United States Office of Solid Waste EPA 550-B-10-001 Environmental Protection and Emergency Response May 2010 Agency www.epa.gov/emergencies LIST OF LISTS Consolidated List of Chemicals Subject to the Emergency Planning and Community Right- To-Know Act (EPCRA), Comprehensive Environmental Response, Compensation and Liability Act (CERCLA) and Section 112(r) of the Clean Air Act • EPCRA Section 302 Extremely Hazardous Substances • CERCLA Hazardous Substances • EPCRA Section 313 Toxic Chemicals • CAA 112(r) Regulated Chemicals For Accidental Release Prevention Office of Emergency Management This page intentionally left blank. TABLE OF CONTENTS Page Introduction................................................................................................................................................ i List of Lists – Conslidated List of Chemicals (by CAS #) Subject to the Emergency Planning and Community Right-to-Know Act (EPCRA), Comprehensive Environmental Response, Compensation and Liability Act (CERCLA) and Section 112(r) of the Clean Air Act ................................................. 1 Appendix A: Alphabetical Listing of Consolidated List ..................................................................... A-1 Appendix B: Radionuclides Listed Under CERCLA .......................................................................... B-1 Appendix C: RCRA Waste Streams and Unlisted Hazardous Wastes................................................ C-1 This page intentionally left blank. LIST OF LISTS Consolidated List of Chemicals -

Environmental Protection Agency Pt. 355, App. A
Environmental Protection Agency Pt. 355, App. A agreement between a State and a sion over which the United States has Tribe, the SERC shall be the entity jurisdiction and Indian Country. identified in the agreement. Threshold planning quantity means, State means any State of the United for a substance listed in Appendices A States, the District of Columbia, the and B of this part, the quantity listed Commonwealth of Puerto Rico, Guam, in the column ‘‘threshold planning American Samoa, the United States quantity’’ for that substance. Virgin Islands, the Northern Mariana [73 FR 65462, Nov. 3, 2008, as amended at 73 Islands, any other territory or posses- FR 76960, Dec. 18, 2008] APPENDIX A TO PART 355—THE LIST OF EXTREMELY HAZARDOUS SUBSTANCES AND THEIR THRESHOLD PLANNING QUANTITIES [Alphabetical Order] Reportable Threshold plan- CAS No. Chemical name Notes quantity * ning quantity (pounds) (pounds) 75–86–5 ................ Acetone Cyanohydrin ................................................. ..................... 10 1,000 1752–30–3 ............ Acetone Thiosemicarbazide ....................................... ..................... 1,000 1,000/10,000 107–02–8 .............. Acrolein ....................................................................... ..................... 1 500 79–06–1 ................ Acrylamide .................................................................. f ................... 5,000 1,000/10,000 107–13–1 .............. Acrylonitrile ................................................................. f .................. -

Commission Implementing Regulation (EU)
L 263/26 EN Official Journal of the European Union 28.9.2012 COMMISSION IMPLEMENTING REGULATION (EU) No 889/2012 of 27 September 2012 amending Annex I to Regulation (EC) No 669/2009 implementing Regulation (EC) No 882/2004 of the European Parliament and of the Council as regards the increased level of official controls on imports of certain feed and food of non-animal origin (Text with EEA relevance) THE EUROPEAN COMMISSION, (4) In particular, for consignments of Brassica oleracea (‘Chinese broccoli’) from China, the information sources Having regard to the Treaty on the Functioning of the European indicate a degree of non-compliance with the relevant Union, safety requirements, thereby warranting the introduction of an increased level of official controls. An entry Having regard to Regulation (EC) No 882/2004 of the European concerning those consignments should therefore be Parliament and of the Council of 29 April 2004 on official included in the list. controls performed to ensure the verification of compliance with feed and food law, animal health and animal welfare (5) In the interest of consistency and clarity of Union legis rules ( 1), and in particular Article 15(5) thereof, lation, it is appropriate to replace Annex I to Regulation (EC) No 669/2009 by the text in the Annex to this Whereas: Regulation. 2 (1) Commission Regulation (EC) No 669/2009 ( ) lays down (6) Regulation (EC) No 669/2009 should therefore be rules concerning the increased level of official controls to amended accordingly. be carried out on imports of feed and food of non- animal origin listed in Annex I thereto (‘the list’), at the (7) The measures provided for in this Regulation are in points of entry into the territories referred to in Annex I accordance with the opinion of the Standing to Regulation (EC) No 882/2004. -

Environmental Protection Agency Pt. 355, App. B
Environmental Protection Agency Pt. 355, App. B [Alphabetical Order] Reportable Threshold plan- CAS No. Chemical name Notes quantity * ning quantity (pounds) (pounds) 5344–82–1 ............ Thiourea, (2-Chlorophenyl)- ....................................... ..................... 100 100/10,000 614–78–8 .............. Thiourea, (2-Methylphenyl)- ....................................... ..................... 500 500/10,000 7550–45–0 ............ Titanium Tetrachloride ................................................ ..................... 1,000 100 584–84–9 .............. Toluene 2,4-Diisocyanate ........................................... ..................... 100 500 91–08–7 ................ Toluene 2,6-Diisocyanate ........................................... ..................... 100 100 110–57–6 .............. Trans-1,4-Dichlorobutene ........................................... ..................... 500 500 1031–47–6 ............ Triamiphos .................................................................. ..................... 500 500/10,000 24017–47–8 .......... Triazofos ..................................................................... ..................... 500 500 76–02–8 ................ Trichloroacetyl Chloride .............................................. ..................... 500 500 115–21–9 .............. Trichloroethylsilane ..................................................... d .................. 500 500 327–98–0 .............. Trichloronate ............................................................... e ................. -

Environmental Protection Agency Pt. 355, App. A
Environmental Protection Agency Pt. 355, App. A Release means any spilling, leaking, the facility is located. In the absence pumping, pouring, emitting, emptying, of a SERC for a State or Indian Tribe, discharging, injecting, escaping, leach- the Governor or the chief executive of- ing, dumping, or disposing into the en- ficer of the tribe, respectively, shall be vironment (including the abandonment the SERC. Where there is a cooperative or discarding of barrels, containers, agreement between a State and a and other closed receptacles) of any Tribe, the SERC shall be the entity hazardous chemical, EHS, or CERCLA identified in the agreement. hazardous substance. Solution means any aqueous or or- Reportable quantity means, for any ganic solutions, slurries, viscous solu- CERCLA hazardous substance, the tions, suspensions, emulsions, or quantity established in Table 302.4 of 40 pastes. CFR 302.4, for such substance. For any State means any State of the United EHS, reportable quantity means the States, the District of Columbia, the quantity established in Appendices A Commonwealth of Puerto Rico, Guam, and B of this part for such substance. American Samoa, the United States Unless and until superseded by regula- Virgin Islands, the Northern Mariana tions establishing a reportable quan- Islands, any other territory or posses- tity for newly listed EHSs or CERCLA sion over which the United States has hazardous substances, a weight of 1 jurisdiction and Indian Country. pound shall be the reportable quantity. Threshold planning quantity means, SERC means the State Emergency for a substance listed in Appendices A Response Commission for the State in and B of this part, the quantity listed which the facility is located except in the column ‘‘threshold planning where the facility is located in Indian quantity’’ for that substance.