19Th Edition, September 2020
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Tidy Towns Competition 2016
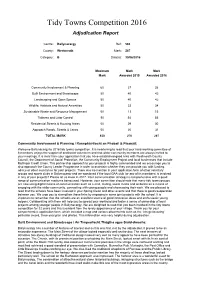
Tidy Towns Competition 2016 Adjudication Report Centre: Ballynacargy Ref: 563 County: Westmeath Mark: 287 Category: B Date(s): 30/06/2016 Maximum Mark Mark Mark Awarded 2015 Awarded 2016 Community Involvement & Planning 60 37 38 Built Environment and Streetscape 50 40 42 Landscaping and Open Spaces 50 40 42 Wildlife, Habitats and Natural Amenities 50 33 34 Sustainable Waste and Resource Management 50 13 15 Tidiness and Litter Control 90 54 55 Residential Streets & Housing Areas 50 29 30 Approach Roads, Streets & Lanes 50 30 31 TOTAL MARK 450 276 287 Community Involvement & Planning / Rannpháirtíocht an Phobail & Pleanáil: Welcome Ballynacargy to 2016 tidy towns competition. It is heartening to read that your hard-working committee of 6 members enjoys the support of dedicated volunteers and that wider community members are always invited to your meetings. It is clear from your application that you have established good links with Westmeath County Council, the Department of Social Protection, the Community Employment Project and local businesses that include Mullingar Credit Union. This partnership approach to your projects is highly commended and we encourage you to also approach the County Leader Programme in order to ascertain whether they can provide you with funding, advice or other assistance for your projects. There was no mention in your application form of other voluntary groups and sports clubs in Ballynacargy and we wondered if the local GAA club (or any of its members) is involved in any of your projects? Please let us know in 2017. Your communication strategy is comprehensive with a good range of communication mediums being used. -

Mount Prospect 20/06/2007 12:09 Page 4
WELCOME TO... MULLINGAR CO. WESTMEATH FOR SALE TO LET gar THE PERFECT MARRIAGE OF OLD WORLD STANDARDS AND MODERN COMFORT Mount Prospect 20/06/2007 12:09 Page 4 elcome to Mount Prospect. This is a small luxury development of just 32 apartments set in one of Mullingar’s most in-demand residential areas, just off the old Dublin Road, two minutes by car to the new M4 motorway and five minutes on foot to Mullingar Town Centre. This is modern luxury apartment living, with all the amenities of Mullingar literally on your doorstep. Mullingar is a vibrant town with easy access to Dublin city; This former market town has become a favourite for those wishing to escape the commotion and expense of the city, while still enjoying the convenience of good supermarkets, pubs, hotels, restaurants, speciality shops, coffee shops, sporting clubs and amenities. Mullingar is surrounded by Loughs Owel, Ennell and Derravaragh, and there are ample opportunities for fishing, sailing and watersports. Mullingar Golf Club has 18 holes of first class parkland golf. Equestrian sports are hugely popular – with stables too numerous to mention in the area and hugely popular events such as the Kilbeggan race meetings. Athletics, Hockey, Rugby and GAA are all served by various well-supported local WELCOME TO MOUNT PROSPECT... sporting clubs. Mullingar is a convenient central location: as well as being an easy drive to Dublin, you have excellent proximity to other main road networks leading to Sligo, Castlebar, Galway, Athlone, Tullamore and Dundalk. From the first moment of your arrival it will be obvious that Mount Prospect, is a little bit different and a little bit special. -

Results 11-2
Mullingar Show Results 2011 RESULTS FOR IPS CLASSES Ring 1 Ridden Hunters 133cms 1st Eamon O Connell’s Ardville Caddy, 2nd Viola Callaghan New Oakes Celebration, 3rd Marianne Hayes Morpark Dora. 143ms 1st Trish Hoey's Woodroyd Gold Label, 2nd P&R Shiel-Mullen's Black Bobby Sparrow, 3rd; D O Sullivan’s Rathclooney Matchmaker. 153cms 1st; Rachel Davies Prince of Dromehily, 2nd; Calvin Nugent's Greggs Cavalier, 3rd; Jade Morton's Highland Scarlet. Champion; Rachel Davies Prince of Dromehily, Res Champion; Calvin Nugent's Greggs Cavalier. Class 4 Lead Rein Hunter Pony type 1st; Liz Palussiere's Muskerry Rolo, 2nd; Clare Winters Dukeshill Cupcake, 3rd; Mairead Ryan's Darcystown Spirit. Class 5 Lead Rein Show Pony type, 1st; Becky O Connor’s Haighead Rising Star. Class 7 Show Ponies combined 1st; Clare Winters Dukeshill Cupcake, 2nd; Nigel Cathers Mountcaulfield First Love, 3rd; Rachel Davies Oakwood Jive Talkin. Class 7A First Ridden 1st; Fiona Hayes, 2nd; Sarah Egan's Ivy Sparkle, 3rd; Megan Jerrad-Dinn Class 7B First year first ridden 1st; Yvonne Byrnes' Paulank Tuffy, 2nd; Melissa O’Connor’s Paulank Tinker Bell, 3rd; Ralph Ternier's Apple Drops. Class 8 Registered Welsh Ridden 1st Becky O’Connor’s Dyfed Piccalo, 2nd; Clare Winters Dukeshill Frodo, 3rd; Clare Winters Dukeshill Cupcake. Class 9 Part Bred Welsh. Ridden 1st; Lucy McCarthy’s Chipmonk, 2nd; Eve Wallace's Starfield Jack. Class 10 Junior Side Saddle 1st Viola Callaghan's New oaks Celebration. Class 11 153cms Working Hunter 1st; Calvin Nugent's Greggs Cavalier, 2nd; Pat Gavin's Ardagh Bobby, 3rd; Sarah Love's Small&Mighty. -

Language Notes on Baronies of Ireland 1821-1891
Database of Irish Historical Statistics - Language Notes 1 Language Notes on Language (Barony) From the census of 1851 onwards information was sought on those who spoke Irish only and those bi-lingual. However the presentation of language data changes from one census to the next between 1851 and 1871 but thereafter remains the same (1871-1891). Spatial Unit Table Name Barony lang51_bar Barony lang61_bar Barony lang71_91_bar County lang01_11_cou Barony geog_id (spatial code book) County county_id (spatial code book) Notes on Baronies of Ireland 1821-1891 Baronies are sub-division of counties their administrative boundaries being fixed by the Act 6 Geo. IV., c 99. Their origins pre-date this act, they were used in the assessments of local taxation under the Grand Juries. Over time many were split into smaller units and a few were amalgamated. Townlands and parishes - smaller units - were detached from one barony and allocated to an adjoining one at vaious intervals. This the size of many baronines changed, albiet not substantially. Furthermore, reclamation of sea and loughs expanded the land mass of Ireland, consequently between 1851 and 1861 Ireland increased its size by 9,433 acres. The census Commissioners used Barony units for organising the census data from 1821 to 1891. These notes are to guide the user through these changes. From the census of 1871 to 1891 the number of subjects enumerated at this level decreased In addition, city and large town data are also included in many of the barony tables. These are : The list of cities and towns is a follows: Dublin City Kilkenny City Drogheda Town* Cork City Limerick City Waterford City Database of Irish Historical Statistics - Language Notes 2 Belfast Town/City (Co. -

Outside Mullingar
OUTSIDE MULLINGAR BY JOHN PATRICK SHANLEY DRAMATISTS PLAY SERVICE INC. OUTSIDE MULLINGAR Copyright © 2014, John Patrick Shanley All Rights Reserved CAUTION: Professionals and amateurs are hereby warned that performance of OUTSIDE MULLINGAR is subject to payment of a royalty. It is fully protected under the copyright laws of the United States of America, and of all countries covered by the International Copyright Union (including the Dominion of Canada and the rest of the British Commonwealth), and of all countries covered by the Pan-American Copyright Convention, the Universal Copyright Convention, the Berne Convention, and of all countries with which the United States has reciprocal copyright relations. All rights, including without limitation professional/amateur stage rights, motion picture, recitation, lecturing, public reading, radio broadcasting, television, video or sound recording, all other forms of mechanical, electronic and digital reproduction, transmission and distribution, such as CD, DVD, the Internet, private and file-sharing networks, information storage and retrieval systems, photocopying, and the rights of translation into foreign languages are strictly reserved. Particular emphasis is placed upon the matter of readings, permission for which must be secured from the Author’s agent in writing. The English language stock and amateur stage performance rights in the United States, its territories, possessions and Canada for OUTSIDE MULLINGAR are controlled exclusively by DRAMATISTS PLAY SERVICE, INC., 440 Park Avenue South, New York, NY 10016. No professional or nonprofessional performance of the Play may be given without obtaining in advance the written permission of DRAMATISTS PLAY SERVICE, INC., and paying the requisite fee. Inquiries concerning all other rights should be addressed to Creative Artists Agency, 405 Lexington Avenue, 19th Floor, New York, NY 10174. -

What Kind of Irish Was Spoken in Westmeath?
What kind of Irish was spoken in Westmeath? AENGUS FINNEGAN By the time of the Gaelic Revival at the end of the 19th century, the Irish language as a vernacular had largely disappeared across Leinster. The small extent of the language which remained was probably confined to the most remote and out-of-the-way townlands, and scattered among a generation who had been largely forgotten by the outside world – with the possible exception of a small part of Co. Louth. It is no wonder, then, that it is primarily to the north, west and southwest areas of Ireland that scholars of the language in all its varying forms have since directed their attention. It is in these regions that the language continued to be spoKen into the 20th century, and indeed continues to be spoKen, though much less extensively than heretofore. This focus, however understandable, has left a large gap in our understanding of the historical distribution of the dialects of Irish across the eastern half of the country. The only means of filling this gap is to carry out a detailed study of the scant remains of the language, as found in word lists, folKlore collections, the later manuscript tradition (if available), everyday speech, and, last but by no means least, in placenames, including both townland and minor names. The great advantage of evidence from placenames to the historial dialectologist is the universal distribution of the placenames themselves. This means that aspects of the language which come to light in the placenames of one area can safely and easily be compared with developments in another. -

Outside Mullingar Script
OUTSIDE MULLINGAR by John Patrick Shanley Contact: George Lane Creative Artists Agency 405 Lexington Ave., 19th Floor Email: [email protected] Phone: 212-277-9000 Fax: 212-277-9099 OUTSIDE MULLINGAR by JPS Scene 1. It’s December, 2008. The sound of cattle, doves, and wind. The bachelor farm kitchen of a cattle and sheep farm outside Killucan, in Ireland. Over the sink, on a shelf, is an old T.V. A turf stove sits on a torn linoleum floor. A small table by a window still has some uncleared dishes. A vinyl chair, with stuffing visible here and there, is set up in a nook created by a staircase. The first of two doors opens and shuts, Off. The second now opens into the kitchen, revealing TONY REILLY, a wily old Irishman in a serviceable dark suit and Greek fishing cap, followed by ANTHONY REILLY, his son. Tony is 75 or so, and his eyes are sly. Anthony is 42, and his eyes of those of an intense dreamer. ANTHONY Jesus, what an experience. My heart feels like a stone. It’s a physical sensation. TONY Why did you do it? That’s what I want to know. ANTHONY The whole half of me cut across the shoulders down is horrible. It’s grief, that’s what it is. TONY We’d be done with it now if it wasn’t for you. ANTHONY Done with what? 2. TONY What do you think? Our obligations. Our social obligations. ANTHONY Obligations? There are no obligations. TONY All that was left to do was goodnight, and sorry for your trouble. -

Roinn Cosanta. Bureau of Military
ROINN COSANTA. BUREAU OF MILITARY HISTORY, 1913-21. STATEMENT BY WITNESS. 1498. DOCUMENT NO. W.S. Witness Michael Murray, 457 Collins Avenue, Swords Road, Whitehall, Dublin. Identity. Lieutenant, Ballynacargy Company, Irish Volunteers. Captain, Ballynacargy Company, I.R.A. Subject. Activities of Ballynacargy Company, I.R.A., 1917-1921. Conditions,if any, Stipulatedby Witness. Nil. File No S.2794. FormB.S.M.2 STAEMENTBY MICHAELMUPRAY, 457, Collins Avenue, Swords Road, Whitehall, Dublin. I was born and reared at Ballynacarrigy, County Westmeath, and received my education at the local school there. Timothy O'Rogan, our school master, was very keen on Irish History and laid special emphasis on this subject so we, the boys of that time, were given a thorough groundworkon which to develop our patriotic sense. In the winter of 1917 a Companyof the Irish Volunteers was started in Ballynacarrigy and I joined this Company. In the Companywe had about fifteen to twenty young men. JamesGaffney was our first CompanyCaptain. On joining we took no oath, nor did we makea declaration of any sort. We had noarms of any sort and used sticks and roughly fashioned woodon guns to train with. The instruction in drill and other subjects was imparted by menwho had served in the British Armyand parades were held at least once per week and sometimesoftener. As well as I can remember the County of Westmeathwas organised as one Battalion area at this time and SeamusO'Mara of Athlone was the Battalion Commandant. Recruiting for the Volunteers then was on a very selective basis. Only manwho camefrom very decent families and whowere endowedwith a strong national outlook were accepted for the force. -

The List of Church of Ireland Parish Registers
THE LIST of CHURCH OF IRELAND PARISH REGISTERS A Colour-coded Resource Accounting For What Survives; Where It Is; & With Additional Information of Copies, Transcripts and Online Indexes SEPTEMBER 2021 The List of Parish Registers The List of Church of Ireland Parish Registers was originally compiled in-house for the Public Record Office of Ireland (PROI), now the National Archives of Ireland (NAI), by Miss Margaret Griffith (1911-2001) Deputy Keeper of the PROI during the 1950s. Griffith’s original list (which was titled the Table of Parochial Records and Copies) was based on inventories returned by the parochial officers about the year 1875/6, and thereafter corrected in the light of subsequent events - most particularly the tragic destruction of the PROI in 1922 when over 500 collections were destroyed. A table showing the position before 1922 had been published in July 1891 as an appendix to the 23rd Report of the Deputy Keeper of the Public Records Office of Ireland. In the light of the 1922 fire, the list changed dramatically – the large numbers of collections underlined indicated that they had been destroyed by fire in 1922. The List has been updated regularly since 1984, when PROI agreed that the RCB Library should be the place of deposit for Church of Ireland registers. Under the tenure of Dr Raymond Refaussé, the Church’s first professional archivist, the work of gathering in registers and other local records from local custody was carried out in earnest and today the RCB Library’s parish collections number 1,114. The Library is also responsible for the care of registers that remain in local custody, although until they are transferred it is difficult to ascertain exactly what dates are covered. -

A Fragment Used by Keating: History (Bk
A Fragment Used by Keating: History (Bk. I., Sec. III) Author(s): Paul Walsh Source: Archivium Hibernicum, Vol. 1 (1912), pp. 1-9, 368b Published by: Catholic Historical Society of Ireland Stable URL: http://www.jstor.org/stable/25485451 . Accessed: 18/06/2014 01:03 Your use of the JSTOR archive indicates your acceptance of the Terms & Conditions of Use, available at . http://www.jstor.org/page/info/about/policies/terms.jsp . JSTOR is a not-for-profit service that helps scholars, researchers, and students discover, use, and build upon a wide range of content in a trusted digital archive. We use information technology and tools to increase productivity and facilitate new forms of scholarship. For more information about JSTOR, please contact [email protected]. Catholic Historical Society of Ireland is collaborating with JSTOR to digitize, preserve and extend access to Archivium Hibernicum. http://www.jstor.org This content downloaded from 195.34.79.208 on Wed, 18 Jun 2014 01:03:32 AM All use subject to JSTOR Terms and Conditions A FRAGMENT USED BY KEATING HISTORY (Bk. L> Sec. III.) fragment edited and translated below is preserved : a marked THE in two manuscripts (i) D, vellum codex, D. iv. 2, in the Royal Irish Academy written in 1300 text is a later at Kilcormac in King's County. Our in hand vacant scribe, on folio 25, which had been left by the original a (2) R, manuscript in the Bodleian Library, Oxford> not so as Rawlinson B. 512. This copy is complete that A shows a verse on eternal in D. -

Athlone to Mullingar
The Old Rail Trail – Westmeath Respect farm animals and wildlife - Dogs to be The Old Rail Trail is a rural route through the heart kept on a short lead and ‘scoop-the-poop’. Keep a of the Irish Midlands, starting in the bustling town safe distance from farm animals. Leave habitats, of Athlone and continuing on a converted stretch plants and animals as you find them. of the Midlands Great Western Railway. In the unlikely event of an emergency dial 999 The 42km journey takes us through rich fertile or 112 to contact Emergency Services. farmland, away from the hustle of towns, in a beautiful rural setting to the market town of Mullingar. The Old Rail Trail forms part of the Lough Owel N4 proposed Galway to Dublin Cycleway, which will be Ireland’s first dedicated inter-city coast to coast route for cyclists. LEAVE NO TRACE Athlone to Be prepared - Although the route is off road, you www.leavenotraceireland.org Royal Canal Greenway still need to take special care at or near all road N52 Mullingar junctions. For more information visit Ensure you have the fitness, equipment and time greenway.westmeathcoco.ie for the walk or the cycle. Check the weather forecast For further local information please contact MULLINGAR and be prepared for changing weather conditions. Westmeath County Council 15 Be considerate of other people - This route is 04493 32000 mixed use - while walking be aware of cyclists 13 14 approaching. Cyclists should use a bell to alert walkers. Park appropriately and do not block WALKING AND entrances or other cars. -

Wardenstown House Killucan, Co
Wardenstown House killucan, co. westmeath Wardenstown House killucan, co. westmeath A superb opportunity to acquire a compact estate incorporating a refurbished Georgian residence surrounded by wonderful old gardens and its own farmland On circa 34.89 ha / 86.22 ac For Sale by Private Treaty Main Residence 3 principal reception rooms w 6 bedrooms (1 en suite) w 2 bathrooms w kitchen and breakfast room w utility w wine cellar w bar w games room w gym w further store rooms Garden Cottage Open plan kitchen, living, dining room w reception room w 2 bedrooms w bathroom House Yard Workshop with loft w log store w garden shed with loft w further storage sheds Stable Yard Two mare and foal stables w 8 stables (2 lofted) w 3 coach houses with loft overhead Farm Buildings Traditional stone farm buildings w large storage shed Lands Lands extend to approximately 34.89 ha / 86.22 ac w laid out in a mix of grassland and forestry w flood lit sand arena Gardens Mature gardens surrounding the house w walled garden Kilbeggan Racecourse is the nearest race course and holds friezes, antique chimney pieces and sash windows with working ISTANCES D festivals and race evenings throughout the year. For keen shutters. Dublin - 69 km horsemen, local hunting packs include the Westmeath Fox Killucan - 3 km Hounds, the Meath Fox Hounds and the Ballymacad Hunt. Kinnegad - 7.5 km ACCOMMODATION There are choices of excellent schools in Mullingar. Wilson’s Mullingar - 18 km Approximately 496.7 sq. m. / 5,346 sq. ft. Hospital is a fee paying day and boarding school in Dublin Airport - 75.5 km The house is approached by a set of stone steps through Multyfarnham (9.8km).