Identification of a Class 3 Aldehyde Dehydrogenase in Human Ofthis
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

The Aldehyde Dehydrogenase ALDH2*2 Allele Exhibits Dominance Over ALDH2*1 in Transduced Hela Cells
The aldehyde dehydrogenase ALDH2*2 allele exhibits dominance over ALDH2*1 in transduced HeLa cells. Q Xiao, … , T Johnston, D W Crabb J Clin Invest. 1995;96(5):2180-2186. https://doi.org/10.1172/JCI118272. Research Article Individuals heterozygous or homozygous for the variant aldehyde dehydrogenase (ALDH2) allele (ALDH2*2), which encodes a protein differing only at residue 487 from the normal protein, have decreased ALDH2 activity in liver extracts and experience cutaneous flushing when they drink alcohol. The mechanisms by which this allele exerts its dominant effect is unknown. To study this effect, the human ALDH2*1 cDNA was cloned and the ALDH2*2 allele was generated by site-directed mutagenesis. These cDNAs were transduced using retroviral vectors into HeLa and CV1 cells, which do not express ALDH2. The normal allele directed synthesis of immunoreactive ALDH2 protein (ALDH2E) with the expected isoelectric point. Extracts of these cells contained increased aldehyde dehydrogenase activity with low Km for the aldehyde substrate. The ALDH2*2 allele directed synthesis of mRNA and immunoreactive protein (ALDH2K), but the protein lacked enzymatic activity. When ALDH2*1-expressing cells were transduced with ALDH2*2 vectors, both mRNAs were expressed and immunoreactive proteins with isoelectric points ranging between those of ALDH2E and ALDH2K were present, indicating that the subunits formed heteromers. ALDH2 activity in these cells was reduced below that of the parental ALDH2*1-expressing cells. Thus, the ALDH2*2 allele is sufficient to cause ALDH2 deficiency in vitro. Find the latest version: https://jci.me/118272/pdf The Aldehyde Dehydrogenase ALDH2*2 Allele Exhibits Dominance over ALDH2*1 in Transduced HeLa Cells Qing Xiao, * Henry Weiner,* Timothy Johnston,* and David W. -

Expression and Characterization of Pantoea CO Dehydrogenase To
www.nature.com/scientificreports OPEN Expression and characterization of Pantoea CO dehydrogenase to utilize CO-containing industrial Received: 31 October 2016 Accepted: 06 February 2017 waste gas for expanding the Published: 14 March 2017 versatility of CO dehydrogenase Eun Sil Choi1,2,*, Kyoungseon Min3,*, Geun-Joong Kim2, Inchan Kwon1 & Yong Hwan Kim3 Although aerobic CO dehydrogenases (CODHs) might be applicable in various fields, their practical applications have been hampered by low activity and no heterologous expression. We, for the first time, could functionally express recombinant PsCODH in E. coli and obtained a highly concentrated recombinant enzyme using an easy and convenient method. Its electron acceptor spectra, optimum −1 conditions (pH 6.5 and 30 °C), and kinetic parameters (kcat of 12.97 s , Km of 0.065 mM, and specific activity of 0.86 Umg−1) were examined. Blast furnace gas (BFG) containing 20% CO, which is a waste gas from the steel-making process, was tested as a substrate for PsCODH. Even with BFG, the recombinant PsCODH retained 88.2% and 108.4% activity compared with those of pure CO and 20% CO, respectively. The results provide not only a promising strategy to utilize CO-containing industrial waste gases as cheap, abundant, and renewable resources but also significant information for further studies about cascade reactions producing value-added chemicals via CO2 as an intermediate produced by a CODH- based CO-utilization system, which would ultimately expand the versatility of CODH. Carbon monoxide (CO), which is a pollutant in the atmosphere, is massively emitted through both natural (e.g., production by plants and volcanic activity) and artificial processes (e.g., incomplete combustion of fuels and industrial processes)1–3. -

The Evolution and Population Genetics of the ALDH2 Locus: Random Genetic Drift, Selection, and Low Levels of Recombination
doi: 10.1046/j.1529-8817.2003.00060.x The evolution and population genetics of the ALDH2 locus: random genetic drift, selection, and low levels of recombination Hiroki Oota1, Andrew J. Pakstis1, Batsheva Bonne-Tamir2, David Goldman3, Elena Grigorenko4, Sylvester L. B. Kajuna5, Nganyirwa J. Karoma5, Selemani Kungulilo6, Ru-Band Lu7, Kunle Odunsi8, Friday Okonofua9, Olga V. Zhukova10, Judith R. Kidd1 and Kenneth K. Kidd1,∗ 1Department of Genetics, Yale University School of Medicine, 333 Cedar Street, P.O. Box 208005, New Haven, CT 06520-8005, USA 2Department of Human Genetics, Sackler School of Medicine, Tel Aviv University, Tel Aviv, Israel 3Laboratory of Neurogenetics, National Institute of Alcohol Abuse and Alcoholism, Rockville, MD 20852, USA 4Department of Psychology, Yale University, New Haven, CT 06520, USA 5The Hubert Kairuki Memorial University, Dar es Salaam, Tanzania 6Muhimbili University College of Health Sciences, Dar es Salaam, Tanzania 7Department of Psychiatry, Tri-Service General hospital, National Defense Medical Center, Taipei, Taiwan, R.O.C. 8Department of Gynecological Oncology, Roswell Park Cancer Institute, Buffalo, NY 14263, USA 9Department of Obstetrics and Gynecology, Faculty of Medicine, University of Benin, Benin City, Nigeria 10N.I. Vavilov Institute of General Genetics RAS, Moscow, Russia Summary The catalytic deficiency of human aldehyde dehydrogenase 2 (ALDH2) is caused by a nucleotide substitution (G1510A; Glu487Lys) in exon 12 of the ALDH2 locus. This SNP,and four non-coding SNPs, including one in the promoter, span 40 kb of ALDH2; these and one downstream STRP have been tested in 37 worldwide populations. Only four major SNP-defined haplotypes account for almost all chromosomes in all populations. -

How Is Alcohol Metabolized by the Body?
Overview: How Is Alcohol Metabolized by the Body? Samir Zakhari, Ph.D. Alcohol is eliminated from the body by various metabolic mechanisms. The primary enzymes involved are aldehyde dehydrogenase (ALDH), alcohol dehydrogenase (ADH), cytochrome P450 (CYP2E1), and catalase. Variations in the genes for these enzymes have been found to influence alcohol consumption, alcohol-related tissue damage, and alcohol dependence. The consequences of alcohol metabolism include oxygen deficits (i.e., hypoxia) in the liver; interaction between alcohol metabolism byproducts and other cell components, resulting in the formation of harmful compounds (i.e., adducts); formation of highly reactive oxygen-containing molecules (i.e., reactive oxygen species [ROS]) that can damage other cell components; changes in the ratio of NADH to NAD+ (i.e., the cell’s redox state); tissue damage; fetal damage; impairment of other metabolic processes; cancer; and medication interactions. Several issues related to alcohol metabolism require further research. KEY WORDS: Ethanol-to acetaldehyde metabolism; alcohol dehydrogenase (ADH); aldehyde dehydrogenase (ALDH); acetaldehyde; acetate; cytochrome P450 2E1 (CYP2E1); catalase; reactive oxygen species (ROS); blood alcohol concentration (BAC); liver; stomach; brain; fetal alcohol effects; genetics and heredity; ethnic group; hypoxia The alcohol elimination rate varies state of liver cells. Chronic alcohol con- he effects of alcohol (i.e., ethanol) widely (i.e., three-fold) among individ- sumption and alcohol metabolism are on various tissues depend on its uals and is influenced by factors such as strongly linked to several pathological concentration in the blood T chronic alcohol consumption, diet, age, consequences and tissue damage. (blood alcohol concentration [BAC]) smoking, and time of day (Bennion and Understanding the balance of alcohol’s over time. -

Bioalcohol Production by a New Synthetic Route in a Hyperthermophilic Archaeon
COMMENTARY COMMENTARY Bioalcohol production by a new synthetic route in a hyperthermophilic archaeon Volker Müller1 Department of Molecular Microbiology & Bioenergetics, Institute of Molecular Biosciences, Johann Wolfgang Goethe University Frankfurt am Main, 60438 Frankfurt, Germany The still growing demand for bioethanol is establishing an electrochemical ion gradient currently met by two microbiological pro- across the cytoplasmic membrane that then cesses. The by far most important process is drives ATP synthesis by a membrane-bound the use of first- (sugar) or second- (lignocel- ATP synthase (5). One widespread, ferre- lulosic biomass) generation raw materials and doxin-fueled ion-translocating enyzme is + yeast as catalysts. Yeasts convert the sugars the ferredoxin:NAD oxidoreductase found via glycolysis to pyruvate, which is decar- in many bacteria and few archaea (6). In P. furiosus boxylated, and the resulting acetaldehyde is , the reduced ferredoxin is oxidized Fig. 1. Pathways for ethanol formation from glucose in then reduced to ethanol by a monofunctional by a different enzyme, a membrane-bound, yeasts (A) and bacteria (B). Adh1, alcoholdehydrogenase ethanol dehydrogenase. The second and in- multisubunit hydrogenase (Mbh) with evolu- 1; AdhE, bifunctional CoA-dependent ethanol/aldehyde dustrially less important is the bacterial pro- tionarysimilaritytothecomplexIofbacterial dehydrogenase. cess, in which pyruvate is oxidized to acetyl- and mitochondrial electron transport chains CoA, which is then reduced by a bifunctional (7). The Mbh is a proton-reducing (hydrogen aldehyde dehydrogenase/ethanol dehydroge- evolving) and sodium ion-extruding mem- used to drive acetate reduction and not the nase (AdhE) to ethanol. This pathway is brane protein complex (8, 9). generation of a chemiosmotic ion gradient, found in many fermenting bacteria (Fig. -
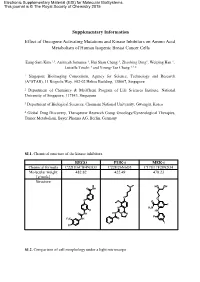
Supplementary Information Effect of Oncogene Activating Mutations And
Electronic Supplementary Material (ESI) for Molecular BioSystems. This journal is © The Royal Society of Chemistry 2015 Supplementary Information Effect of Oncogene Activating Mutations and Kinase Inhibitors on Amino Acid Metabolism of Human Isogenic Breast Cancer Cells Eung-Sam Kim 1,3, Animesh Samanta 1, Hui Shan Cheng 1, Zhaobing Ding1, Weiping Han 1, Luisella Toschi 4 and Young-Tae Chang 1,2,* 1 Singapore Bioimaging Consortium, Agency for Science, Technology and Research (A*STAR), 11 Biopolis Way, #02-02 Helios Building, 138667, Singapore 2 Department of Chemistry & MedChem Program of Life Sciences Institute, National University of Singapore, 117543, Singapore. 3 Department of Biological Sciences, Chonnam National University, Gwangju, Korea 4 Global Drug Discovery, Therapeutic Research Group Oncology/Gynecological Therapies, Tumor Metabolism, Bayer Pharma AG, Berlin, Germany SI.1. Chemical structure of the kinase inhibitors. REGO PI3K-i MEK-i Chemical formula C22H16ClF4N3O3 C22H26N6O3 C17H17F2IN2O4 Molecular weight 482.82 422.49 478.23 [g/mole] Structure N HO OH O NH O O O O O F N H2N F HN O NH HN N N F F3C NH O N I Cl SI.2. Comparison of cell morphology under a light microscope Scale bar: 100 µm 10x objective lens at Ti microscope (Nikon) SI.3. Scheme of the experimental method for metabolite extraction SI.4. Gradient profile of HPLC run. A : Buffer solution B: Mobile phase H O Time 2 (40 mM NaHPO4, (MeOH/ACN/H2O, Flow rate pH7.8 at RT) 45:45:10, v/v/v) (ml/min) (min) (%) (%) (%) 0 100 0 0 1.0 3 90 10 0 1.0 15 87 13 0 1.0 25 70 30 0 1.0 37 65 35 0 1.0 43 58 42 0 1.0 52 43 57 0 1.0 55 0 100 0 1.0 60 0 0 100 1.0 65 to 80 100 0 0 1.0 SI.5. -

A Novel Bifunctional Aldehyde/Alcohol Dehydrogenase Mediating Ethanol Formation from Acetyl-Coa in Hyperthermophiles
A novel bifunctional aldehyde/alcohol dehydrogenase mediating ethanol formation from acetyl-CoA in hyperthermophiles Qiang Wang Jiangsu University Chong Sha Jiangsu University Hongcheng Wang Jiangsu University Kesen Ma University of Waterloo Juergen Wiegel University of Georgia Abd El-Fatah Abomohra Tanta University Weilan Shao ( [email protected] ) Jiangsu University Research Keywords: Acetyl-CoA reduction, Bifunctional aldehyde/alcohol dehydrogenase, Ethanol fermentation, Hyperthermophiles, Thermotoga neapolitana Posted Date: March 15th, 2020 DOI: https://doi.org/10.21203/rs.3.rs-17223/v1 License: This work is licensed under a Creative Commons Attribution 4.0 International License. Read Full License Page 1/15 Abstract Background: Hyperthermophilic fermentation at temperatures above 80 °C allows in situ product removal to mitigate the ethanol toxicity, and reduces microbial contamination without autoclaving/cooling of feedstock. Many species of Thermotoga grow at temperatures up to 90 °C, and have enzymes to degrade and utilize lignocelluloses, which provide advantages for achieving consolidated processes of cellulosic ethanol production. However, no CoA-dependent aldehyde dehydrogenase (CoA-Aldh) from any hyperthermophiles has been documented in literature so far. The pyruvate ferredoxin oxidoreductases from hyperthermophiles have pyruvate decarboxylase activity, which convert about 2% and 98% of pyruvate to acetaldehyde and acetyl-CoA (ac-CoA), respectively. Acetyl-CoA can be converted to acetic acid, if there is no CoA-Aldh -

Effect of the Allelic Variants of Aldehyde
REVIEW Effect of the allelic variants of aldehyde dehydrogenase ALDH2*2 and alcohol dehydrogenase ADH1B*2 on blood acetaldehyde concentrations Giia-Sheun Peng1 and Shih-Jiun Yin2* 1Department of Neurology, Tri-Service General Hospital, 325 Chenggong Road Section 2, Taipei 114, Taiwan 2Department of Biochemistry, National Defense Medical Center, 161 Minchuan East Road Section 6, Taipei 114, Taiwan *Correspondence to: Tel: þ886 2 8792 3100 ext 18800; Fax: þ886 2 8792 4818; E-mail: [email protected] Date received (in revised form): 30th July 2008 Abstract Alcoholism is a complex behavioural disorder. Molecular genetics studies have identified numerous candidate genes associated with alcoholism. It is crucial to verify the disease susceptibility genes by correlating the pinpointed allelic variations to the causal phenotypes. Alcohol dehydrogenase (ADH) and aldehyde dehydrogenase (ALDH) are the principal enzymes responsible for ethanol metabolism in humans. Both ADH and ALDH exhibit functional polymorphisms among racial populations; these polymorphisms have been shown to be the important genetic determinants in ethanol metabolism and alcoholism. Here, we briefly review recent advances in genomic studies of human ADH/ALDH families and alcoholism, with an emphasis on the pharmacogenetic consequences of venous blood acetaldehyde in the different ALDH2 genotypes following the intake of various doses of ethanol. This paper illustrates a paradigmatic example of phenotypic verifications in a protective disease gene for substance abuse. Keywords: alcohol dehydrogenase, aldehyde dehydrogenase, single nucleotide polymorphism, alcoholism, ethanol metab- olism, blood acetaldehyde Introduction Alcohol dehydrogenase (ADH) and aldehyde dehy- exposure and the concentrations of ethanol and its drogenase (ALDH) are the principal enzymes respon- metabolite acetaldehyde attained in body fluids and sible for hepatic metabolism of ethanol.1 Human tissue within that period. -

ALDH2) in Neuropathology and Neurodegeneration
Review Article 111 The Role of Mitochondrial Aldehyde Dehydrogenase 2 (ALDH2) in Neuropathology and Neurodegeneration Che-Hong Chen, Amit U. Joshi, Daria Mochly-Rosen* Abstract- Aldehydes-induced toxicity has been implicated in many neurodegenerative diseases. Exposure to reactive aldehydes from (1) alcohol and food metabolism; (2) environmental pollutants, including car, factory exhausts, smog, pesticides, herbicides; (3) metabolism of neurotransmitters, amino acids and (4) lipid peroxidation of biological membrane from excessive ROS, all contribute to ‘aldehydic load’ that has been linked to the pathology of neurodegenerative diseases. In particular, the α, β-unsaturated aldehydes derived from lipid peroxidation, 4-hydroxynonenal (4-HNE), DOPAL (MAO product of dopamine), malondialdehyde, acrolein and acetaldehyde, all readily form chemical adductions with proteins, DNA and lipids, thus causing neurotoxicity. Mitochondrial aldehyde dehydrogenase 2 (ALDH 2) is a major aldehyde metabolizing enzyme that protects against deleterious aldehyde buildup in brain, a tissue that has a particularly high mitochondrial content. In this review, we highlight the deleterious effects of increased aldehydic load in the neuropathology of ischemic stroke, Alzheimer’s disease and Parkinson’s disease. We also discuss evidence for the association between ALDH2 deficiency, a common East Asian- specific mutation, and these neuropathologies. A novel class of small molecule aldehyde dehydrogenase activators (Aldas), represented by Alda-1, reduces neuronal cell death in models of ischemic stroke, Alzheimer’s disease and Parkinson’s disease. Together, these data suggest that reducing aldeydic load by enhancing the activity of aldehyde dehydrogenases, such as ALDH2, represents as a therapeutic strategy for neurodegenerative diseases. Acta Neurol Taiwan 2016;25:111-123 (6-8) INTRODUCTION phosphorylation . -
![Molecular Characterization of the Fatty Alcohol Oxidation Pathway for Wax-Ester Mobilization in Germinated Jojoba Seeds1[W]](https://docslib.b-cdn.net/cover/2047/molecular-characterization-of-the-fatty-alcohol-oxidation-pathway-for-wax-ester-mobilization-in-germinated-jojoba-seeds1-w-3802047.webp)
Molecular Characterization of the Fatty Alcohol Oxidation Pathway for Wax-Ester Mobilization in Germinated Jojoba Seeds1[W]
Molecular Characterization of the Fatty Alcohol Oxidation Pathway for Wax-Ester Mobilization in Germinated Jojoba Seeds1[W] Alex S. Rajangam2, Satinder K. Gidda, Christian Craddock3, Robert T. Mullen, John M. Dyer, and Peter J. Eastmond* School of Life Sciences, University of Warwick, Coventry, Warwickshire CV4 7AL, United Kingdom (A.S.R., C.C.); Department of Molecular and Cellular Biology, University of Guelph, Guelph, Ontario, Canada N1G 2W1 (S.K.G., R.T.M.); United States Department of Agriculture-Agricultural Research Service, United States Arid-Land Agricultural Research Center, Maricopa, Arizona 85238 (J.M.D.); and Department of Crop Biology and Plant Science, Rothamsted Research, Harpenden,HertfordshireAL52JQ,United Kingdom (P.J.E.) Jojoba (Simmondsia chinensis) is the only plant species known to use liquid wax esters (WEs) as a primary seed storage reserve. Upon germination, WE hydrolysis releases very-long-chain fatty alcohols, which must be oxidized to fatty acids by the sequential action of a fatty alcohol oxidase (FAO) and a fatty aldehyde dehydrogenase (FADH) before they can be b-oxidized. Here, we describe the cloning and characterization of genes for each of these two activities. Jojoba FAO and FADH are 52% and 68% identical to Arabidopsis (Arabidopsis thaliana) FAO3 and ALDH3H1, respectively. The genes are expressed most strongly in the cotyledons of jojoba seedlings following germination, but transcripts can also be detected in vegetative tissues. Proteomic analysis indicated that the FAO and FADH proteins can be detected on wax bodies, but they localized to the endoplasmic reticulum when they were expressed as amino-terminal green fluorescent protein fusions in tobacco (Nicotiana tabacum) leaves. -

Aldehyde-Alcohol Dehydrogenase And/Or Thiolase Overexpression
ARTICLE Aldehyde–Alcohol Dehydrogenase and/or Thiolase Overexpression Coupled With CoA Transferase Downregulation Lead to Higher Alcohol Titers and Selectivity in Clostridium acetobutylicum Fermentations Ryan Sillers,1 Mohab Ali Al-Hinai,2 Eleftherios T. Papoutsakis1,3 1Department of Chemical and Biological Engineering, Northwestern University, Evanston, Illinois 60208; telephone: 302-831-8376; fax: 302-831-4841; e-mail: [email protected] 2Biology Department, College of Science, Sultan Qaboos University, Muscat, Oman 3Department of Chemical Engineering & The Delaware Biotechnology Institute, University of Delaware, 15 Innovation Way, Newark, DE 19716. Received 11 May 2008; revision received 14 July 2008; accepted 15 July 2008 Published online 22 July 2008 in Wiley InterScience (www.interscience.wiley.com). DOI 10.1002/bit.22058 sion in strains with asRNA CoAT downregulation did not ABSTRACT: Metabolic engineering (ME) of Clostridium significantly alter product formation thus suggesting that a acetobutylicum has led to increased solvent (butanol, acet- more complex metabolic engineering strategy is necessary to one, and ethanol) production and solvent tolerance, thus enhance the intracellular butyryl-CoA pool and reduce the demonstrating that further efforts have the potential to acetyl-CoA pool in order to achieve improved butanol titers create strains of industrial importance. With recently devel- and selectivity. oped ME tools, it is now possible to combine genetic Biotechnol. Bioeng. 2009;102: 38–49. modifications and thus implement more advanced ME ß 2008 Wiley Periodicals, Inc. strategies. We have previously shown that antisense RNA KEYWORDS: Clostridium acetobutylicum; metaboli engi- (asRNA)-based downregulation of CoA transferase (CoAT, neering; thiolase; flux analysis; acetyl-CoA; butyryl-CoA the first enzyme in the acetone-formation pathway) results in increased butanol to acetone selectivity, but overall reduced butanol yields and titers. -

RT² Profiler PCR Array (Rotor-Gene® Format) Mouse Amino Acid Metabolism I
RT² Profiler PCR Array (Rotor-Gene® Format) Mouse Amino Acid Metabolism I Cat. no. 330231 PAMM-129ZR For pathway expression analysis Format For use with the following real-time cyclers RT² Profiler PCR Array, Rotor-Gene Q, other Rotor-Gene cyclers Format R Description The Mouse Amino Acid Metabolism I RT² Profiler PCR Array profiles the expression of 84 key genes important in biosynthesis and degradation of functional amino acids. Of the 20 amino acids required for protein synthesis, six of them (arginine, cysteine, glutamine, leucine, proline, and tryptophan), collectively known as the functional amino acids, regulate key metabolic pathways involved in cellular growth, and development, as well as other important biological processes such as immunity and reproduction. For example, leucine activates mTOR signaling and increases protein synthesis, leading to lymphocyte proliferation. Therefore, a lack of leucine can compromise immune function. Metabolic pathways interrelated with the biosynthesis and degradation of these amino acids include vitamin and cofactor biosynthesis (such as SAM or S-Adenosyl Methionine) as well as neurotransmitter metabolism (such as glutamate). This array includes genes for mammalian functional amino acid metabolism as well as genes involved in methionine metabolism, important also for nutrient sensing and sulfur metabolism. Using realtime PCR, you can easily and reliably analyze the expression of a focused panel of genes involved in functional amino acid metabolism with this array. For further details, consult the RT² Profiler PCR Array Handbook. Shipping and storage RT² Profiler PCR Arrays in the Rotor-Gene format are shipped at ambient temperature, on dry ice, or blue ice packs depending on destination and accompanying products.