Plant Functional Group Diversity As a Mechanism for Invasion Resistance
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Propagation of Colorado Natives at Little Valley©
224 Combined Proceedings International Plant Propagators’ Society, Volume 58, 2008 Propagation of Colorado Natives at Little Valley © Brian Core Little Valley Wholesale Nursery, 13022 E. 136th Ave., Brighton, Colorado 80601 U.S.A. Email: [email protected] INTRODUCTION Little Valley Wholesale Nursery was established in 1979 with 15 acres of field and container stock and a staff of four people. Over the years, it has expanded to en- compass 141 acres and a peak-season staff of more than 150 people. The plant palette now includes more than 500 taxa of perennials, 275 taxa of shrubs, and over 130 taxa of trees. The area served by Little Valley’s distribution system includes Wyoming, Colorado, New Mexico, and Arizona. Little Valley’s motto “The Rocky Mountain Standard” expresses the commitment to be the best wholesale plant dis- tributor in the western region. Native plants have been an important part of Little Valley’s plant mix for many years. Native plants are ideal for low-input sustainable landscapes. The Denver Metro area receives 8–15 inches of rain per year; in contrast, many eastern cities receive 50 or even 60 inches of annual precipitation. Colorado has a rapidly expanding population that threatens to deplete the water supply within a few decades. Many native plants require no supplemental water when established in the landscape; water that is not wasted on bluegrass lawns is water that can be used for human consumption. Native plants also require less frequent fertilizer and pesticide appli- cations; overuse of fertilizer and pesticide can lead to contamination of groundwater supplies with carcinogens. -

An Ethnobotany of Mount Rushmore National Memorial
University of Massachusetts Amherst ScholarWorks@UMass Amherst Landscape Architecture & Regional Planning Masters Projects Landscape Architecture & Regional Planning Summer 2019 An Ethnobotany of Mount Rushmore National Memorial Meredith Savage University of Massachusetts Amherst Follow this and additional works at: https://scholarworks.umass.edu/larp_ms_projects Part of the Landscape Architecture Commons Savage, Meredith, "An Ethnobotany of Mount Rushmore National Memorial" (2019). Landscape Architecture & Regional Planning Masters Projects. 92. Retrieved from https://scholarworks.umass.edu/larp_ms_projects/92 This Article is brought to you for free and open access by the Landscape Architecture & Regional Planning at ScholarWorks@UMass Amherst. It has been accepted for inclusion in Landscape Architecture & Regional Planning Masters Projects by an authorized administrator of ScholarWorks@UMass Amherst. For more information, please contact [email protected]. AN ETHNOBOTANY OF MOUNT RUSHMORE NATIONAL MEMORIAL Department of Landscape Architecture and Regional Planning University of Massachusetts Amherst Master of Regional Planning Project Meredith S. Savage July 2019 © Copyright by Meredith S. Savage 2019 All Rights Reserved AN ETHNOBOTANY OF MOUNT RUSHMORE NATIONAL MEMORIAL Department of Landscape Architecture and Regional Planning University of Massachusetts Amherst Master of Regional Planning Project Meredith S. Savage July 2019 Approved as to style and content by: __________________________________________ Dr. Elizabeth Brabec, -

Riverside State Park
Provisonal Report Rare Plant and Vegetation Survey of Riverside State Park Pacific Biodiversity Institute 2 Provisonal Report Rare Plant and Vegetation Survey of Riverside State Park Peter H. Morrison [email protected] George Wooten [email protected] Juliet Rhodes [email protected] Robin O’Quinn, Ph.D. [email protected] Hans M. Smith IV [email protected] January 2009 Pacific Biodiversity Institute P.O. Box 298 Winthrop, Washington 98862 509-996-2490 Recommended Citation Morrison, P.H., G. Wooten, J. Rhodes, R. O’Quinn and H.M. Smith IV, 2008. Provisional Report: Rare Plant and Vegetation Survey of Riverside State Park. Pacific Biodiversity Institute, Winthrop, Washington. 433 p. Acknowledgements Diana Hackenburg and Alexis Monetta assisted with entering and checking the data we collected into databases. The photographs in this report were taken by Peter Morrison, Robin O’Quinn, Geroge Wooten, and Diana Hackenburg. Project Funding This project was funded by the Washington State Parks and Recreation Commission. 3 Executive Summary Pacific Biodiversity Institute (PBI) conducted a rare plant and vegetation survey of Riverside State Park (RSP) for the Washington State Parks and Recreation Commission (WSPRC). RSP is located in Spokane County, Washington. A large portion of the park is located within the City of Spokane. RSP extends along both sides of the Spokane River and includes upland areas on the basalt plateau above the river terraces. The park also includes the lower portion of the Little Spokane River and adjacent uplands. The park contains numerous trails, campgrounds and other recreational facilities. The park receives a tremendous amount of recreational use from the nearby population. -

Vegetation of the Fort Riley Military Reservation Kansas
VVEGETATION OF THE FFORT RRILEY MMILITARY RRESERVATION,, KKANSAS Craig C. Freeman1 and Jennifer M. Delisle2 1R. L. McGregor Herbarium & Kansas Biological Survey University of Kansas, 2045 Constant Avenue, Lawrence, KS 66047-3729 [email protected] 2Kansas Biological Survey University of Kansas, 2101 Constant Avenue, Lawrence, KS 66047-3759 [email protected] VEGETATION OF THE FORT RILEY MILITARY RESERVATION, KANSAS Cover Photo: Gray Copper (Lycaena dione) on Sullivant’s milkweed (Asclepias sullivantii); Fort Riley Military Reservation, Geary County, Kansas. Photo by Craig C. Freeman, 2003. Report submitted February 27, 2004 Citation: Freeman, C. C. and J. M. Delisle. 2004. Vegetation of the Fort Riley Military Reservation, Kansas. Open-file Report No. 119. Kansas Biological Survey. Lawrence, KS. 110 pp. Table of Contents LIST OF FIGURES.......................................................................................................................................................................... II LIST OF TABLES ...........................................................................................................................................................................III ACKNOWLEDGMENTS .............................................................................................................................................................IV ABSTRACT....................................................................................................................................................................................... -

Color and Common Name Prairie Plant List
Native or Likely Color Common Name Scientific Name Family Introduced to See Orange N Butterflyweed Asclepias tuberosa var. interior Asclepiadaceae X Orange N Michigan Lily Lilium michiganense Liliaceae Pink I Alsike Clover Trifolium hybridum Fabaceae X Pink N American Vetch Vicia americana Fabaceae X Pink I Bouncing Bet Saponaria officinalis Caryophyllaceae X Pink I Bull Thistle Cirsium vulgare Asteraceae X Pink I Canada Thistle Cirsium arvense Asteraceae X Pink N Canada Tick Trefoil Desmodium canadense Fabaceae X Pink I Catnip Nepeta cataria Lamiaceae X Pink N Common Milkweed Asclepias syriaca Asclepiadaceae X Pink I Crownvetch Coronilla varia Fabaceae X Pink N Dotted Blazing Star Liatris punctata var. punctata Asteraceae X Pink N Great Blazing Star Liatris pycnostachya var. pycnostachya Asteraceae X Pink N Heart-Leaved Four O'Clock Mirabilis nyctaginea Nyctagenaceae X Pink N Hill's Thistle Cirsium pumilum var. hillii Asteraceae Pink N Northern Plains Blazing Star Liatris ligulistylis Asteraceae Pink N Obedient Plant Physostegia virginiana var. virginiana Lamiaceae X Pink N Prairie Phlox Phlox pilosa var. fulgida Polemoniaceae X Pink N Prairie Rose Rosa arkansana Rosaceae X Pink N Prairie Smoke Geum triflorum Rosaceae X Pink N Prairie Wild Onion Allium stellatum Liliaceae Pink I Rabbit's Foot Clover Trifolium arvense Fabaceae X Pink I Red Clover Trifolium pratense Fabaceae X Pink N Rough Blazing Star Liatris aspera Asteraceae X Pink I Spotted Knapweed Centaurea stoebe subsp. micranthos Asteraceae X Pink N Spreading Dogbane Apocynum androsaemifolium Apocynaceae X Pink N Swamp Milkweed Asclepias incarnata var. incarnata Asclepiadaceae X Pink I Tartarian Honeysuckle Lonicera tatarica Caprifoliaceae X Pink N Wild Bergamot Monarda fistulosa Lamiaceae X Pink N Wild Garlic Allium canadense var. -
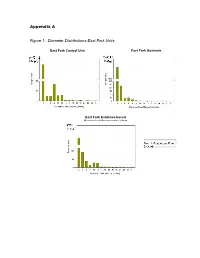
Appendix a Figure 1: Diameter Distributions-East Fork Units
Appendix A Figure 1: Diameter Distributions-East Fork Units East Fork Control Unit East Fork Goshawk Guidelines Unit East Fork Evidence-based Ecological Restoration Unit Figure 2: Diameter Distributions-Redondo Units Redondo Control Unit Redondo Goshawk Guidelines Unit Redondo Evidence-based Ecological Restoration Unit Figure 3: FlamMap Simulated Crown Fire Behavior: East Fork Project Area 20 mph Southwest Wind 25 mph 30 mph 35 mph 40 mph Map Legend Crown Fire Activity N No crown fire Passive crown activity fire (torching) Active crown fire (spreading) Important Note: Expected Crown Fire Behavior by Percent The 97th percentile of low fuel of Project Area moistures for June from 34 No Crown Passive years of data from the Kaibab Fire Crown Fire Active National Forest, Arizona were used in all simulations. This Windspeed Activity (torching) Crown Fire 20 79.9 0.9 19.2 input represents potential 25 71.2 1.9 26.9 extremes of fire weather during the peak of the fire 30 64.9 2.9 32.3 season, not average fire 35 57.3 4.7 38.0 season conditions. 40 48.7 9.4 42.0 Figure 4: FlamMap Simulated Crown Fire Behavior: Redondo Project Area 20 mph Southwest Wind 25 mph 30 mph 35 mph 40 mph Map Legend Crown Fire Activity N Highway 4 Valles Caldera No crown fire Passive crown activity entrance road fire (torching) Active crown fire (spreading) Important Note: Expected Crown Fire Behavior by Percent The 97th percentile of low fuel of Project Area moistures for June from 34 Passive years of data from the Kaibab No Crown Crown Fire Active Crown National Forest, Arizona were Windspeed Fire Activity (torching) Fire used in all simulations. -

List of Plants for Great Sand Dunes National Park and Preserve
Great Sand Dunes National Park and Preserve Plant Checklist DRAFT as of 29 November 2005 FERNS AND FERN ALLIES Equisetaceae (Horsetail Family) Vascular Plant Equisetales Equisetaceae Equisetum arvense Present in Park Rare Native Field horsetail Vascular Plant Equisetales Equisetaceae Equisetum laevigatum Present in Park Unknown Native Scouring-rush Polypodiaceae (Fern Family) Vascular Plant Polypodiales Dryopteridaceae Cystopteris fragilis Present in Park Uncommon Native Brittle bladderfern Vascular Plant Polypodiales Dryopteridaceae Woodsia oregana Present in Park Uncommon Native Oregon woodsia Pteridaceae (Maidenhair Fern Family) Vascular Plant Polypodiales Pteridaceae Argyrochosma fendleri Present in Park Unknown Native Zigzag fern Vascular Plant Polypodiales Pteridaceae Cheilanthes feei Present in Park Uncommon Native Slender lip fern Vascular Plant Polypodiales Pteridaceae Cryptogramma acrostichoides Present in Park Unknown Native American rockbrake Selaginellaceae (Spikemoss Family) Vascular Plant Selaginellales Selaginellaceae Selaginella densa Present in Park Rare Native Lesser spikemoss Vascular Plant Selaginellales Selaginellaceae Selaginella weatherbiana Present in Park Unknown Native Weatherby's clubmoss CONIFERS Cupressaceae (Cypress family) Vascular Plant Pinales Cupressaceae Juniperus scopulorum Present in Park Unknown Native Rocky Mountain juniper Pinaceae (Pine Family) Vascular Plant Pinales Pinaceae Abies concolor var. concolor Present in Park Rare Native White fir Vascular Plant Pinales Pinaceae Abies lasiocarpa Present -

Hare-Footed Locoweed,Oxytropis Lagopus
COSEWIC Assessment and Status Report on the Hare-footed Locoweed Oxytropis lagopus in Canada THREATENED 2014 COSEWIC status reports are working documents used in assigning the status of wildlife species suspected of being at risk. This report may be cited as follows: COSEWIC. 2014. COSEWIC assessment and status report on the Hare-footed Locoweed Oxytropis lagopus in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. xi + 61 pp. (www.registrelep-sararegistry.gc.ca/default_e.cfm). Previous report(s): COSEWIC. 1995. COSEWIC status report on the Hare-footed Locoweed Oxytropis lagopus in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. 24 pp. Smith, Bonnie. 1995. COSEWIC status report on the Hare-footed Locoweed Oxytropis lagopus in Canada. Committee on the Status of Endangered Wildlife in Canada. Ottawa. 24 pp. Production note: C COSEWIC would like to acknowledge Juanita Ladyman for writing the status report on the Hare-footed Locoweed (Oxytropis lagopus) in Canada, prepared under contract with Environment Canada. This report was overseen and edited by Bruce Bennett, Co-chair of the Vascular Plant Specialist Subcommittee. For additional copies contact: COSEWIC Secretariat c/o Canadian Wildlife Service Environment Canada Ottawa, ON K1A 0H3 Tel.: 819-953-3215 Fax: 819-994-3684 E-mail: COSEWIC/[email protected] http://www.cosewic.gc.ca Également disponible en français sous le titre Ếvaluation et Rapport de situation du COSEPAC sur L’oxytrope patte-de-lièvre (Oxytropis lagopus) au Canada. Cover illustration/photo: Hare-footed Locoweed — Photo credit: Cheryl Bradley (with permission). Her Majesty the Queen in Right of Canada, 2014. -

VASCULAR PLANTS of MINNESOTA a Checklist and Atlas
VASCULAR PLANTS of MINNESOTA This page intentionally left blank VASCULAR PLANTS of MINNESOTA A Checklist and Atlas Gerald B. Ownbey and Thomas Morley UNIVERSITY OF MINNESOTA MINNEAPOLIS • LONDON The University of Minnesota Press gratefully acknowledges the generous assistance provided for the publication of this book by the Margaret W. Harmon Fund Minnesota Department of Transportation Minnesota Landscape Arboretum Minnesota State Horticultural Society Olga Lakela Herbarium Fund—University of Minnesota—Duluth Natural Heritage Program of the Minnesota Department of Natural Resources Copyright © 1991 by the Regents of the University of Minnesota. First paperback printing 1992 All rights reserved. No part of this publication may be reproduced, stored in a retrieval system, or transmitted, in any form or by any means, electronic, mechanical, photocopying, recording, or otherwise, without the prior written permission of the publisher. Published by the University of Minnesota Press 2037 University Avenue Southeast, Minneapolis, MN 55455 Printed in the United States of America on acid-free paper Library of Congress Cataloging-in-Publication Data Ownbey, Gerald B., 1916- Vascular plants of Minnesota : a checklist and atlas / Gerald B. Ownbey and Thomas Morley. p. cm. Includes bibliographical references and index. ISBN 0-8166-1915-8 1. Botany-Minnesota. 2. Phytogeography—Minnesota— Maps. I. Morley, Thomas. 1917- . II. Title. QK168.096 1991 91-2064 582.09776-dc20 CIP The University of Minnesota is an equal-opportunity educator and employer. Contents Introduction vii Part I. Checklist of the Vascular Plants of Minnesota 1 Pteridophytes 3 Gymnosperms 6 Angiosperms 7 Appendix 1. Excluded names 81 Appendix 2. Tables 82 Part II. Atlas of the Vascular Plants of Minnesota 83 Index of Generic and Common Names 295 This page intentionally left blank Introduction The importance of understanding the vegetation of al distributional comments. -

Annotated Checklist of Vascular Flora, Cedar Breaks National
National Park Service U.S. Department of the Interior Natural Resource Program Center Annotated Checklist of Vascular Flora Cedar Breaks National Monument Natural Resource Technical Report NPS/NCPN/NRTR—2009/173 ON THE COVER Peterson’s campion (Silene petersonii), Cedar Breaks National Monument, Utah. Photograph by Walter Fertig. Annotated Checklist of Vascular Flora Cedar Breaks National Monument Natural Resource Technical Report NPS/NCPN/NRTR—2009/173 Author Walter Fertig Moenave Botanical Consulting 1117 W. Grand Canyon Dr. Kanab, UT 84741 Editing and Design Alice Wondrak Biel Northern Colorado Plateau Network P.O. Box 848 Moab, UT 84532 February 2009 U.S. Department of the Interior National Park Service Natural Resource Program Center Fort Collins, Colorado The Natural Resource Publication series addresses natural resource topics that are of interest and applicability to a broad readership in the National Park Service and to others in the management of natural resources, including the scientifi c community, the public, and the NPS conservation and environmental constituencies. Manuscripts are peer-reviewed to ensure that the information is scientifi cally credible, technically accurate, appropriately written for the intended audience, and is designed and published in a professional manner. The Natural Resource Technical Report series is used to disseminate the peer-reviewed results of scientifi c studies in the physical, biological, and social sciences for both the advancement of science and the achievement of the National Park Service’s mission. The reports provide contributors with a forum for displaying comprehensive data that are often deleted from journals because of page limitations. Current examples of such reports include the results of research that addresses natural resource management issues; natural resource inventory and monitoring activities; resource assessment reports; scientifi c literature reviews; and peer- reviewed proceedings of technical workshops, conferences, or symposia. -

Protocol Information
Protocol Information Dave Skinner PMC Farm Manager Pullman Plant Materials Center Room 104 Hulbert Hall WSU Pullman, Washington 99163-6211 509-335-9689 509-335-2940 Fax [email protected] Family Scientific Name: Asteraceae Family Common Name: Sunflower Scientific Name: Microseris nutans (Hook.) Schulz-Bip. ' ' Common Synonym: ' ' Common Name: Nodding microseris Species Code: MINU Ecotype: Paradise Creek drainage near Pullman, Washington. General Distribution: British Columbia and northwestern US east to Montana and South Dakota and south to Colorado and California. Mean annual precipitation range is from 16- 60 inches (USDA NRCS 2006). In eastern Washington it is usually found in mesic open grassland and open Ponderosa pine forest. Known Invasiveness: Propagation Goal: Plants Propagation Method: Seed Product Type: Container (plug) Stock Type: Time To Grow: 14 Months Target Specifications: Tight root plug in container. Propagule Collection: Fruit is an achene which ripens in June. It is collected when the pappus begins to expand. Seed is brown in color and wind disseminated, so must be collected before it blows away. Seed maturity is indeterminant and daily collections are needed to maximize volume. Seed is stored in paper bags at room temperature until cleaned. Seed should be stored loosely in bags. Compressing the seed with the attached pappus increases difficulty during cleaning. We determined 215,692 seeds/ lb for this ecotype. Propagule Processing: Seed is rubbed lightly, then passed thru a hand screen and rubbed again to separate the pappus, then cleaned with an air column separator. Clean seed is stored in controlled conditions at 40 degrees Fahrenheit and 40% relative humidity. -

Dancing Rock Property Friends of the Columbia River Gorge Dalles Mt
Dancing Rock Property Friends of the Columbia River Gorge Dalles Mt. Road Klickitat County, WA T2N R14E S18, 19 Compiled by Paul Slichter. Updated November 21, 2010 Flora Northwest- http://science.halleyhosting.com Contact the Friends of the Columbia River Gorge for access to this site & to be sure you won't trespass onto neighboring property. Common Name Scientific Name Family Big-leaf Maple Acer macrophyllum Aceraceae Poison Oak Toxicodendron (diversilobum ?) Anacardiaceae Bur Chervil Anthriscus caucalis Apiaceae Gorman's Salt and Pepper Lomatium gormanii Apiaceae Pungent Desert Parsley Lomatium grayii Apiaceae Biscuitroot Lomatium macrocarpum Apiaceae Barestem Desert Parsley Lomatium nudicaule Apiaceae Slender-fruit Desert Parsley Lomatium leptocarpum ? Apiaceae Gairdner's Yampah Perideridia gairdneri Apiaceae Narrow-leaf Milkweed Asclepias fascicularis Asclepediaceae Yarrow Achillea millefolium Asteraceae Large-flowered Agoseris Agoseris grandiflora Asteraceae Annual Agoseris Agoseris heterophylla Asteraceae Low Pussytoes Antennaria dimorpha Asteraceae Carey's Balsamroot Balsamorhiza careyana? Asteraceae Tumbleweed Knapweed Centaurea diffusa Asteraceae Rush Skeletonweed Chondrilla juncea Asteraceae Chicory Cichorum intybus Asteraceae Wavyleaf Thistle Cirsium undulatum Asteraceae Gold Stars Crocidium multicaule Asteraceae Gray Rabbitbrush Ericameria nauseosa (v. speciosa?) Asteraceae Columbia Goldenweed Ericameria resinosa Asteraceae Threadleaf Fleabane Erigeron filifolius Asteraceae Cushion Fleabane Erigeron poliospermus Asteraceae