Determining the Glycaemic Index of Standard and High-Sugar Rodent Diets in C57BL/6 Mice
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Dietary Glycemic Index and Load and the Risk of Type 2 Diabetes: Assessment of Causal Relations
nutrients Review Dietary Glycemic Index and Load and the Risk of Type 2 Diabetes: Assessment of Causal Relations Geoffrey Livesey 1,* , Richard Taylor 1, Helen F. Livesey 1, Anette E. Buyken 2, David J. A. Jenkins 3,4,5,6, Livia S. A. Augustin 4,7, John L. Sievenpiper 3,4,5,6, Alan W. Barclay 8, Simin Liu 9, Thomas M. S. Wolever 3,4, Walter C. Willett 10, Furio Brighenti 11 , Jordi Salas-Salvadó 12,13 , Inger Björck 14, Salwa W. Rizkalla 15, Gabriele Riccardi 16, Carlo La Vecchia 17 , Antonio Ceriello 18, Antonia Trichopoulou 19, Andrea Poli 20, Arne Astrup 21 , Cyril W. C. Kendall 3,4,22, Marie-Ann Ha 23 , Sara Baer-Sinnott 24 and Jennie C. Brand-Miller 25 1 Independent Nutrition Logic Ltd, 21 Bellrope Lane, Wymondham NR180QX, UK; [email protected] (R.T.); [email protected] (H.F.L.) 2 Institute of Nutrition, Consumption and Health, Faculty of Natural Sciences, Paderborn University, 33098 Paderborn, Germany; [email protected] 3 Departments of Nutritional Science and Medicine, Faculty of Medicine, University of Toronto, Toronto, ON M5S 1A8, Canada; [email protected] (D.J.A.J.); [email protected] (J.L.S.); [email protected] (T.M.S.W.); [email protected] (C.W.C.K.) 4 Clinical Nutrition and Risk Factor Modification Centre, St. Michael’s Hospital, Toronto, ON M5C 2T2, Canada; [email protected] 5 Division of Endocrinology and Metabolism, Department of Medicine, St. Michael’s Hospital, Toronto, ON M5C 2T2, Canada 6 Li Ka Shing Knowledge Institute, St. -

Properties of Maltodextrins and Glucose Syrups in Experiments in Vitro and in the Diets of Laboratory Animals, Relating to Dental Health
Downloaded from British Journal of Nutrition (2000), 84, 565±574 565 https://www.cambridge.org/core Properties of maltodextrins and glucose syrups in experiments in vitro and in the diets of laboratory animals, relating to dental health T. H. Grenby* and M. Mistry . IP address: Department of Oral Medicine and Pathology, GKT Dental Institute, Guy's Hospital, London SE1 9RT, UK (Received 5 July 1999 ± Revised 13 December 1999 ± Accepted 26 January 2000) 170.106.33.42 The objective of the study was to examine the cariogenic potentials of maltodextrins and glucose , on syrups (two glucose polymers derived from starch) using a range of techniques in vitro and in 01 Oct 2021 at 02:19:26 laboratory animals. The experimental methods used were: (1) measurement of acid production from glucose syrups and maltodextrins by human dental plaque micro-organisms; (2) evaluation of the role salivary a-amylase in degrading oligosaccharides (degree of polymerisation .3) in the glucose polymers, estimating the products by HPLC; (3) assessment of the fermentability of trioses relative to maltose; (4) measurement of dental caries levels in three large-scale studies in laboratory rats fed on diets containing the glucose polymers. It was found that acid production from the glucose polymers increased as their higher saccharide content fell. Salivary a-amylase , subject to the Cambridge Core terms of use, available at rapidly degraded the oligosaccharides (degree of polymerisation .3), mainly to maltose and maltotriose. In the presence of oral micro-organisms, maltotriose took longer to ferment than maltose, but by the end of a 2 h period the total amount of acid produced was the same from both. -

GRAINS of TRUTH for DIABETES by Jeanne Brown, RD,CDE, LD
GRAINS OF TRUTH FOR DIABETES By Jeanne Brown, RD,CDE, LD Avoiding pasta to lower your blood sugar? Wondering how to identify whole grains? Does the glycemic index (GI) of grains matter? Here are some grains of truth for diabetes. Whole grains provide fiber, vitamins, minerals, antioxidants and in general have a lower GI which reduces the risk of diabetes. Researchers have found a 30 % lower risk of diabetes with high fiber diets from grains. People who eat at least 2 servings per week of brown rice have a 11% lower risk of Type 2 Diabetes than those that eat less. The risk of getting Type 2 Diabetes was 2 ½ times greater for people who had the highest glycemic load and lowest fiber intake in the study. The GI measures how a carbohydrate- containing food raises blood sugar. The GI ranges from 0 to 100. The higher the number, the more likely it will raise blood sugars. The GI is also affected by how the food is processed, how it is cooked and the ripeness of fruit. Pasta cooked just until it is firm and chewy has a lower GI than overcooked pasta. A ripe banana will spike blood sugar more than an unripe banana. Tips: Look for the word “WHOLE” grain. “Whole” ensures they used the entire grain which includes the fiberous bran, and nutrient dense germ. “Wheat bread” or “wheat flour” is not necessarily whole grain unless it states “whole wheat" in the beginning of the ingredient list. Products such as: 100% whole wheat bread, shredded wheat cereal and Triscuit crackers are 100 % whole grains. -

Fiber Fit for All!
fiber fit for all! what is Fibersol-2? Fibersol®-2 digestion-resistant maltodextrin is a soluble corn fiber that acts as a low-calorie bulking agent containing 90 percent dietary fiber. It can be used with minimal formulation adjustments in a variety of food applications to maintain Fiber for Health or improve a product’s desired attributes. Even at significant levels, Fibersol-2 Fibersol®-2, digestion resistant doesn’t affect taste or viscosity. Fibersol-2, digestion resistant maltodextrin, is a maltodextrin, is a low viscosity soluble spray-dried powder produced by a proprietary method of controlled enzymatic dietary fiber that clinical research has hydrolysis of cornstarch. It has numerous starch linkages that remain undigested indicated helps support or maintain by enzymes of the human digestive tract. It is recognized as GRAS (generally intestinal regularity. Clinical studies show recognized as safe) by the Food and Drug Administration and certified Kosher that Fibersol-2 helps to relieve occasional and Pareve by the Orthodox Union. A variety of functional, physical, and sensory constipation and select studies show that attributes of Fibersol-2, digestion resistant maltodextrin, will bring opportunities it improves stool consistency. to food and beverage applications. Studies show that, when taken with a meal, Fibersol-2, digestion resistant Physical Characteristics maltodextrin, may attenuate the rise Color: Off-white powder; clear, transparent in 10% solution; in serum glucose following the meal. resists both enzymatic and non-enzymatic browning. Fibersol-2 has the potential to reduce Soluble Dietary Fiber Flavor: No flavor, clean peak postprandial blood glucose and Fibersol®-2 is a 90 percent minimum dry solids Solubility: Water soluble up to 70% (w/w) at 20° C insulin levels that are within the normal basis soluble dietary fiber (in accordance with Dispersibility: Excellent range in healthy individuals. -

14608 Federal Register / Vol
14608 Federal Register / Vol. 63, No. 58 / Thursday, March 26, 1998 / Rules and Regulations Street, Kansas City, Missouri 64106; DEPARTMENT OF TRANSPORTATION DEPARTMENT OF HEALTH AND telephone: (816) 426±3408. HUMAN SERVICES Federal Aviation Administration SUPPLEMENTARY INFORMATION: On Food and Drug Administration January 20, 1998, the FAA published in 14 CFR Part 71 Federal Register a direct final rule; 21 CFR Part 184 request for comments which modified [Airspace Docket No. 97±ACE±20] the Class E airspace at Columbus [Docket No. 91G±0451] Municipal Airport, NE (FR Doc. 98± Amendment to Class E Airspace; Direct Food Substances Affirmed as 1230, 63 FR 2887, Airspace Docket No. Marshall Army Airfield, Fort Riley, KS Generally Recognized as Safe; 97±ACE±32). The effective date of the Maltodextrin Derived From Rice Starch document is amended to coincide with AGENCY: Federal Aviation the chart change date. After careful Administration, DOT. AGENCY: Food and Drug Administration, review of all available information HHS. ACTION: Direct final rule; confirmation of related to the subject presented above, ACTION: Final rule. effective date. the FAA has determined that air safety and the public interest require adoption SUMMARY: The Food and Drug SUMMARY: This notice confirms the of the rule. The FAA has determined Administration (FDA) is amending its effective date of a direct final rule which that these corrections will not change regulations to affirm that maltodextrin revises Class E airspace at Marshall the meaning of the action nor add any derived from rice starch is generally Army Airfield, Fort Riley, KS. additional burden on the public beyond recognized as safe (GRAS). -

Effect of Glycemic Index and Glycemic Load on Type 2 Diabetes Mellitus
International Journal of Health Sciences and Research www.ijhsr.org ISSN: 2249-9571 Review Article Effect of Glycemic Index and Glycemic Load on Type 2 Diabetes Mellitus Towhid Hasan1, Marjia Sultana1, Lincon Chandra Shill1, Nafisa Habib Purba1, Sara Sultana2 1Department of Food Technology and Nutrition Science, Noakhali Science and Technology University, Noakhali-3814, Bangladesh 2Department of Pharmacy, University of Dhaka, Dhaka-1000, Bangladesh Corresponding Author: Marjia Sultana ABSTRACT Worldwide diabetes mellitus especially type 2 diabetes mellitus (T2DM) has become a common and rapidly growing chronic non-communicable disease with potentially devastating complications, posing major public health and socioeconomic challenges. The role of glycemic index (GI) and glycemic load (GL) in the control of the T2DM remain controversial. However, a number of mechanisms have been proposed through which dietary GI and/or GL can reduce the risk of T2DM, but all are suggestive. So, the present review is aimed to investigate the current understanding of the effect of GI and/or GL on the risk of T2DM.PubMed, Google Scholar, Cochrane library and several other databases were searched up to December 2018 to identify and select relevant studies. Research conducted using human are only considered eligible. Among the eighteen prospective cohort studies, eight have found no significant association of dietary GI and/or GL with T2DM risk, six studies revealed an association and four studies identified positive association only with GI but not with the GL. Among the ten interventional studies, only two indicated that the risk of T2DM increased with the increment of GI and GL. In conclusion, research on the glycemic response of foods needs to be expanded in order to get a broad and clear knowledge which will provide assurance that how dietary GI and/or GL have play role on the risk of T2DM. -

Maltodextrin
Global Supplier Of Plant Based Ingredients Maltodextrin Applications Specifications Food industry Appearance pH • Cakes, cookies, frostings, granola bars, dry mixes yellow amorphous powder 4.0-6.5 and ice creams • Soups, sauces, creams and salad dressings Moisture DE • Nutritional and sports drinks 10.0% max max 20 Ash SO2 0.5% max max 0.0025% Properties Packaging & Shelf life DE Value • 25 kg bags Browning High • 50 lbs • FIBC Hygroscopicity/humectant properties High Plasticity High Minimum of 24 months Sweetness High after date of production, Solubility High in closed packing. Best before expiration date Osmolality High on packaging. Molecular weight Low Viscocity Low Cohesiveness Low Flim-forming properties Low Prevention of large sugar-crystal information Low Contact Meelunie B.V. - Viñoly Tower, 18th floor, Claude Debussylaan 40, 1082 MD Amsterdam P.O. Box 10102, 1001 EC Amsterdam, The Netherlands +31.20.530.6530 | [email protected] | www.meelunie.com Global Supplier Of Plant Based Ingredients Your eye on the market since 1867 General information Our services Meelunie was founded in The Netherlands in 1867. • Competitive prices Since then we have become a key global supplier in • Consistent quality & guaranteed supply starches, sweeteners and proteins in more than 90 • Multiple origins sourcing countries. Our brands are internationally recognised as • Local support a standard of excellence and reliability. With supply and • Market intelligence advisory distribution networks around the world, we are • Multiple logistic solutions "Your -

Dietary Glycemic Index, Glycemic Load, Fiber, Simple Sugars, and Insulin Resistance the Inter99 Study
Pathophysiology/Complications ORIGINAL ARTICLE Dietary Glycemic Index, Glycemic Load, Fiber, Simple Sugars, and Insulin Resistance The Inter99 study 1,2 1,4 CATHRINE LAU, MSC OLUF PEDERSEN, MD, DMSC he rising prevalence of disorders as- 1,2 1 KRISTINE FÆRCH, MSC BENDIX CARSTENSEN, MSC sociated with insulin resistance, 1,3 3 CHARLOTTE GLUMER¨ , MD, PHD TORBEN JØRGENSEN, MD, DMSC 2 1 including type 2 diabetes, cardio- NGE ETENS MSC, PHD NUT ORCH OHNSEN MD, DMSC T I T , K B -J , vascular disease, and the metabolic syn- drome, is commonly attributed to changes over time in dietary patterns and physical activity levels (1–4). Preventive strategies against these disorders, therefore, often in- OBJECTIVE — To examine the relationship between daily glycemic index, daily glycemic corporate dietary recommendations. How- load, simple sugars, dietary fiber, and the prevalence of a measure of insulin resistance in 30- to 60-year-old nondiabetic Danish men and women. ever, only few observational studies have investigated the independent effects of car- RESEARCH DESIGN AND METHODS — The Inter99 study is a nonpharmacological bohydrate-related dietary factors and obe- intervention study. We used baseline data and examined cross-sectional associations between sity on the degree of insulin resistance. The carbohydrate-related dietary factors and an estimate of insulin resistance in 5,675 subjects at official carbohydrate-related recommen- 30–60 years. The dietary intake was estimated from a self-administered food frequency ques- dations are high intake of fiber-rich car- tionnaire, and insulin resistance was estimated using the homeostasis model assessment of bohydrates (Ͼ55 E% [energy percent] insulin resistance (HOMA-IR). -

Low–Glycemic Index Diets in the Management of Diabetes a Meta-Analysis of Randomized Controlled Trials
Clinical Care/Education/Nutrition ORIGINAL ARTICLE Low–Glycemic Index Diets in the Management of Diabetes A meta-analysis of randomized controlled trials 1 2 JENNIE BRAND-MILLER, PHD PETER PETOCZ, PHD ticularly with regard to the glycemic in- 2 3 SUSAN HAYNE, BSC STEPHEN COLAGIURI, MD dex (GI) of foods (5). Current dietary recommendations emphasize the quantity rather than the quality of carbohydrate, despite the fact that carbohydrate source and nature pro- OBJECTIVE — The use of diets with low glycemic index (GI) in the management of diabetes foundly influence postprandial glycemia is controversial, with contrasting recommendations around the world. We performed a meta- (6,7). Research on GI indicates that even analysis of randomized controlled trials to determine whether low-GI diets, compared with conventional or high-GI diets, improved overall glycemic control in individuals with diabetes, as when foods contain the same amount of assessed by reduced HbA or fructosamine levels. carbohydrate (i.e., carbohydrate ex- 1c changes), there are up to fivefold differ- RESEARCH DESIGN AND METHODS — Literature searches identified 14 studies, ences in glycemic impact (8). In addition, comprising 356 subjects, that met strict inclusion criteria. All were randomized crossover or several prospective observational studies parallel experimental design of 12 days’ to 12 months’ duration (mean 10 weeks) with modifi- have found that the overall GI and glyce- cation of at least two meals per day. Only 10 studies documented differences in postprandial mic load (GI ϫ g carbohydrate) of the glycemia on the two types of diet. diet, but not total carbohydrate content, are independently related to the risk of RESULTS — Low-GI diets reduced HbA by 0.43% points (CI 0.72–0.13) over and above 1c developing type 2 diabetes (9,10), cardio- that produced by high-GI diets. -

PDS ELN Vivinal®-GOS-Powder-Maltodextrin.Pdf
Product data sheet Vivinal® GOS Powder Maltodextrin Vivinal GOS Powder Maltodextrin is a galacto-oligosaccharide ingredient with maltodextrin as a carrier. Scientific studies have shown positive effects of oligosaccharides, among which galacto-oligosaccharides, on growth of bifidobacteria1,2, stool consistency3,4, bowel function and transit time5,6, support of natural defences7-10 and absorption of calcium and iron11-13. Product characteristics Packaging Vivinal GOS Powder Maltodextrin is an ingredient Vivinal GOS Powder Maltodextrin is packed in a multiple containing galacto-oligosaccharides (GOS). It is produced layered paperbag with a polyethylene inner liner with net from high quality lactose using a proprietary enzymatic content of 25kg. production technology. This product is spay-dried with maltodextrin and is perfectly suitable for dry blending. Shelf life and storage conditions Vivinal GOS Powder Maltodextrin is stable during long- Application term storage. Both the oligosaccharide content and the Vivinal GOS Powder Maltodextrin is used world-wide as an product characteristics making Vivinal GOS Powder ingredient for standard and premium infant formulas, Maltodextrin unique remain unchanged (no degradation) follow-on formulas, growing-up milk and medical nutrition for at least 18 months when stored under clean, dry and applications. Scientific studies have shown positive effects dark conditions and separated from strongly odorous of oligosaccharides, among which GOS, on growth of materials. bifidobacteria1,2, stool consistency3,4, bowel function and transit time5,6, support of natural defences7-10 and mineral absorption11-13. Next to oligosaccharides, Vivinal GOS Powder Maltodextrin also contains maltodextrin, which is a widely used source of carbohydrates in the infant nutrition industry. The taste of Vivinal GOS Powder Maltodextrin can be characterized as sweet. -

Glycemic Index
GLYCEMIC INDEX High GI Foods = GI of 70+ (Try to avoid. Keep as a reward.) Medium GI = GI of 55 to 69. (Use caution. Avoid when possible.) Low GI = GI of 0 to 54. (This is your target zone. Choose foods FRUITS Cherries 22 Grapefruit 25 Prunes 29 Apricots, dried 30 Apple 38 Peach, canned in juice 38 Pear, fresh 38 Plum 39 Strawberries 40 Orange, Navel 42 Peach, fresh 42 Pear, canned 43 Grapes 46 Mango 51 Banana 52 Fruit Cocktail 55 Papaya 56 Raisins 56 Apricots, fresh 57 Kiwi 58 Figs, dried 61 Apricots, canned 64 Cantaloupe 65 Pineapple, fresh 66 Watermelon 72 Dates 103 Food GI Value GRAINS & RICE Barley, pearled 25 Converted, White 38 Reference: http://www.glycemicedge.com/glycemic-index-chart/ Long grain, White 44 Buckwheat 54 Brown 55 Basmati 58 Couscous 65 Cornmeal 68 Aborio 69 Short grain, White 72 Instant, White 87 Wild rice 87 Glutinous (Sticky) 98 BEANS & PEAS GI Value Chana Dal 8 Chickpeas, dried 28 Kidney Beans, dried 28 Lentils 29 Lima Beans (frozen) 32 Yellow Split Peas 32 Chickpeas, canned 42 Blackeyed Peas, canned 42 Baked Beans 48 Kidney Beans, canned 52 VEGETABLES Broccoli 10 Cabbage 10 Lettuce 10 Mushrooms 10 Onions 10 Red Peppers 10 Carrots 49 Green peas 48 Corn, fresh 60 Beets 64 Pumpkin 75 Parsnips 97 DAIRY Yogurt, artificially sweetened 14 Whole milk 31 Skim milk 32 Yogurt, sweetened 33 Ice cream, premium 38 Reference: http://www.glycemicedge.com/glycemic-index-chart/ Ice cream, low fat 43 SWEETNER Glucose 96 Fructose 22 Lactose 46 Sucrose (white sugar) 64 Brown sugar 64 Barley malt syrup 42 Brown rice syrup 25 Raw honey 30 Agave syrup 15 High fructose corn syrup 62 Stevia less than 1 Sugar cane juice 43 Evaporated cane juice 55 Maple syrup 54 Black strap molasses 55 The materials and content contained on this form are for general holistic nutrition information only to help support and enhance the body’s own healing properties and are not intended to be a substitute for professional medical advice, diagnosis or treatment for any medical condition. -
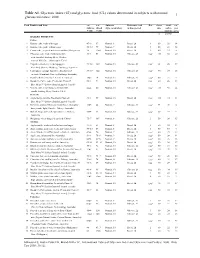
And Glycemic Load (GL) Values Determined in Subjects with Normal 1 Glucose Tolerance: 2008
Table A1. Glycemic index (GI) and glycemic load (GL) values determined in subjects with normal 1 glucose tolerance: 2008 Food Number and Item GI2 GI2 Subjects Reference food Ref. Serve Avail. GL3 (Glucose (Bread (type & number) & time period Size carbo- per = 100) = 100) hydrate serve g g/serve BAKERY PRODUCTS Cakes 1 Banana cake, made with sugar 47±8 67 Normal, 8 Bread, 2h 1 60 29 14 2 Banana cake, made without sugar 55±10 79 Normal, 7 Bread, 2h 1 60 22 12 3 Carrot cake, prepared with coconut flour (Philippines) 36 52±3 Normal, 10 Bread, 2h 2 60 23 8 4 Chocolate cake made from packet mix 38±3 54 Normal, 10 Glucose, 2h UO4 111 52 20 with chocolate frosting (Betty Crocker, General Mills Inc., Minneapolis, USA) 5 Cupcake, strawberry-iced (Squiggles, 73±12 104 Normal, 10 Glucose, 2h UO4 38 26 19 Farmland, Grocery Holdings, Tooronga, Australia) 6 Lamingtons (sponge dipped in chocolate and 87±17 124 Normal, 10 Glucose, 2h UO4 50 29 25 coconut) (Farmland, Grocery Holdings, Australia) 7 Pound cake 0% (Bimbo S.A de C.V, Mexico) 38±5 54 Normal, 12 Glucose, 2h UO4 60 25 9 8 Raspberry Coffee cake, President's Choice® 50±4 71 Normal, 10 Bread, 2h UO5 60 26 13 Blue Menu™ (Loblaw Brands Limited, Canada) 9 Vanilla cake, made from packet mix with 42±4 60 Normal, 10 Glucose, 2h UO4 111 58 24 vanilla frosting (Betty Crocker, USA) Desserts 10 Apple Berry crumble, President's Choice® 41±3 59 Normal, 10 Bread, 2h UO5 165 34 14 Blue Menu™ (Loblaw Brands Limited, Canada) 11 Bavarian (mousse filling on biscuit base), Chocolate 31±5 44 Normal, 9 Glucose, 2h UO6