Low–Glycemic Index Diets in the Management of Diabetes a Meta-Analysis of Randomized Controlled Trials
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Dietary Glycemic Index and Load and the Risk of Type 2 Diabetes: Assessment of Causal Relations
nutrients Review Dietary Glycemic Index and Load and the Risk of Type 2 Diabetes: Assessment of Causal Relations Geoffrey Livesey 1,* , Richard Taylor 1, Helen F. Livesey 1, Anette E. Buyken 2, David J. A. Jenkins 3,4,5,6, Livia S. A. Augustin 4,7, John L. Sievenpiper 3,4,5,6, Alan W. Barclay 8, Simin Liu 9, Thomas M. S. Wolever 3,4, Walter C. Willett 10, Furio Brighenti 11 , Jordi Salas-Salvadó 12,13 , Inger Björck 14, Salwa W. Rizkalla 15, Gabriele Riccardi 16, Carlo La Vecchia 17 , Antonio Ceriello 18, Antonia Trichopoulou 19, Andrea Poli 20, Arne Astrup 21 , Cyril W. C. Kendall 3,4,22, Marie-Ann Ha 23 , Sara Baer-Sinnott 24 and Jennie C. Brand-Miller 25 1 Independent Nutrition Logic Ltd, 21 Bellrope Lane, Wymondham NR180QX, UK; [email protected] (R.T.); [email protected] (H.F.L.) 2 Institute of Nutrition, Consumption and Health, Faculty of Natural Sciences, Paderborn University, 33098 Paderborn, Germany; [email protected] 3 Departments of Nutritional Science and Medicine, Faculty of Medicine, University of Toronto, Toronto, ON M5S 1A8, Canada; [email protected] (D.J.A.J.); [email protected] (J.L.S.); [email protected] (T.M.S.W.); [email protected] (C.W.C.K.) 4 Clinical Nutrition and Risk Factor Modification Centre, St. Michael’s Hospital, Toronto, ON M5C 2T2, Canada; [email protected] 5 Division of Endocrinology and Metabolism, Department of Medicine, St. Michael’s Hospital, Toronto, ON M5C 2T2, Canada 6 Li Ka Shing Knowledge Institute, St. -

GRAINS of TRUTH for DIABETES by Jeanne Brown, RD,CDE, LD
GRAINS OF TRUTH FOR DIABETES By Jeanne Brown, RD,CDE, LD Avoiding pasta to lower your blood sugar? Wondering how to identify whole grains? Does the glycemic index (GI) of grains matter? Here are some grains of truth for diabetes. Whole grains provide fiber, vitamins, minerals, antioxidants and in general have a lower GI which reduces the risk of diabetes. Researchers have found a 30 % lower risk of diabetes with high fiber diets from grains. People who eat at least 2 servings per week of brown rice have a 11% lower risk of Type 2 Diabetes than those that eat less. The risk of getting Type 2 Diabetes was 2 ½ times greater for people who had the highest glycemic load and lowest fiber intake in the study. The GI measures how a carbohydrate- containing food raises blood sugar. The GI ranges from 0 to 100. The higher the number, the more likely it will raise blood sugars. The GI is also affected by how the food is processed, how it is cooked and the ripeness of fruit. Pasta cooked just until it is firm and chewy has a lower GI than overcooked pasta. A ripe banana will spike blood sugar more than an unripe banana. Tips: Look for the word “WHOLE” grain. “Whole” ensures they used the entire grain which includes the fiberous bran, and nutrient dense germ. “Wheat bread” or “wheat flour” is not necessarily whole grain unless it states “whole wheat" in the beginning of the ingredient list. Products such as: 100% whole wheat bread, shredded wheat cereal and Triscuit crackers are 100 % whole grains. -

A Dozen Tips for Better Blood Sugar Control: Glycemic Index And
A Dozen Tips for Better Blood Sugar Control: Glycemic Index and Glycemic Load Human bodies depend on a steady supply of glucose (blood sugar) as their principal fuel, in order for muscles to stretch and contract, nerves to fire, brains to function—and so much more. Glucose comes from carbohydrates, so the quality and quantity of carbohydrates we eat hugely impacts our energy levels and overall health. Too little glucose, and we starve many bodily functions (especially the brain, which uses 11–20% of the glucose we produce). Too much, and our body scrambles to produce enough insulin to process all that blood sugar—and we may develop heart disease, eye, kidney, and nerve damage. Ideally, our food delivers a steady stream of just the right amount of glucose. But how do we distinguish foods, meals, and diets that raise our blood sugar too high and too fast from those that dole out their fuel slowly and steadily to support good health? Understanding glycemic index, glycemic load and glycemic response can help. Glycemic Index (GI) ranks the quality of individual carbohydrate-rich foods on a scale of 1–100 by measuring how glucose levels rise after someone eats an amount of that food containing 50 grams of carbohydrate. Foods with a low GI score (under 55) provide steady fuel to support energy levels and overall health, while those with a high GI score (70 and up) are likely to provide an unhealthy quick rush of blood sugar followed by a sharp crash. Glycemic Load (GL) combines quality and quantity, allowing us to rank how the typical serving size of a food affects blood sugar. -

Effect of Glycemic Index and Glycemic Load on Type 2 Diabetes Mellitus
International Journal of Health Sciences and Research www.ijhsr.org ISSN: 2249-9571 Review Article Effect of Glycemic Index and Glycemic Load on Type 2 Diabetes Mellitus Towhid Hasan1, Marjia Sultana1, Lincon Chandra Shill1, Nafisa Habib Purba1, Sara Sultana2 1Department of Food Technology and Nutrition Science, Noakhali Science and Technology University, Noakhali-3814, Bangladesh 2Department of Pharmacy, University of Dhaka, Dhaka-1000, Bangladesh Corresponding Author: Marjia Sultana ABSTRACT Worldwide diabetes mellitus especially type 2 diabetes mellitus (T2DM) has become a common and rapidly growing chronic non-communicable disease with potentially devastating complications, posing major public health and socioeconomic challenges. The role of glycemic index (GI) and glycemic load (GL) in the control of the T2DM remain controversial. However, a number of mechanisms have been proposed through which dietary GI and/or GL can reduce the risk of T2DM, but all are suggestive. So, the present review is aimed to investigate the current understanding of the effect of GI and/or GL on the risk of T2DM.PubMed, Google Scholar, Cochrane library and several other databases were searched up to December 2018 to identify and select relevant studies. Research conducted using human are only considered eligible. Among the eighteen prospective cohort studies, eight have found no significant association of dietary GI and/or GL with T2DM risk, six studies revealed an association and four studies identified positive association only with GI but not with the GL. Among the ten interventional studies, only two indicated that the risk of T2DM increased with the increment of GI and GL. In conclusion, research on the glycemic response of foods needs to be expanded in order to get a broad and clear knowledge which will provide assurance that how dietary GI and/or GL have play role on the risk of T2DM. -

Dietary Glycemic Index, Glycemic Load, Fiber, Simple Sugars, and Insulin Resistance the Inter99 Study
Pathophysiology/Complications ORIGINAL ARTICLE Dietary Glycemic Index, Glycemic Load, Fiber, Simple Sugars, and Insulin Resistance The Inter99 study 1,2 1,4 CATHRINE LAU, MSC OLUF PEDERSEN, MD, DMSC he rising prevalence of disorders as- 1,2 1 KRISTINE FÆRCH, MSC BENDIX CARSTENSEN, MSC sociated with insulin resistance, 1,3 3 CHARLOTTE GLUMER¨ , MD, PHD TORBEN JØRGENSEN, MD, DMSC 2 1 including type 2 diabetes, cardio- NGE ETENS MSC, PHD NUT ORCH OHNSEN MD, DMSC T I T , K B -J , vascular disease, and the metabolic syn- drome, is commonly attributed to changes over time in dietary patterns and physical activity levels (1–4). Preventive strategies against these disorders, therefore, often in- OBJECTIVE — To examine the relationship between daily glycemic index, daily glycemic corporate dietary recommendations. How- load, simple sugars, dietary fiber, and the prevalence of a measure of insulin resistance in 30- to 60-year-old nondiabetic Danish men and women. ever, only few observational studies have investigated the independent effects of car- RESEARCH DESIGN AND METHODS — The Inter99 study is a nonpharmacological bohydrate-related dietary factors and obe- intervention study. We used baseline data and examined cross-sectional associations between sity on the degree of insulin resistance. The carbohydrate-related dietary factors and an estimate of insulin resistance in 5,675 subjects at official carbohydrate-related recommen- 30–60 years. The dietary intake was estimated from a self-administered food frequency ques- dations are high intake of fiber-rich car- tionnaire, and insulin resistance was estimated using the homeostasis model assessment of bohydrates (Ͼ55 E% [energy percent] insulin resistance (HOMA-IR). -

POTATOES and the GLYCEMIC INDEX How to Reduce the GI of Potatoes and Gain the Benefits of Resistant Starch
POTATOES AND THE GLYCEMIC INDEX How to reduce the GI of potatoes and gain the benefits of resistant starch Potatoes are a highly nutritious wholefood. They contribute a range of nutrients associated with good health, including fibre, vitamin B6, vitamin C, potassium and phytonutrients. You might also be surprised to know that potatoes can have a lower glycemic index (GI) than you might think. What is the Glycemic Index? The GI is a measure of the speed at which a food makes blood sugar rise. AT A GLANCE GI is calculated by measuring the change in blood glucose (BGL) over a The GI of potatoes is two-hour period after eating a 50 gram carbohydrate portion of a single food relative to the change in blood glucose after eating a 50g carbohydrate influenced by: portion of a reference food (glucose or white bread). • Variety of potato (and its carbohydrate, fibre and When it is not possible to measure this directly, GI tables are used to provide polyphenol content) an estimate of BGL response, however, there are large fluctuations in BGL response to foods both within and between individuals. • Serving size The lower a food’s GI, the slower BGLs rise after eating that food. There are • Cooking method/preparation many factors affecting GI in nuts and potatoes. GI less than 55 is considered and storage low, 56-69 moderate and 70+ high. • Fibre and resistant starch content (higher amounts = To better understand a food’s effect on BGLs, you need to know how quickly lower GI, leave the skin on) its glucose enters the bloodstream and how much glucose per serving it can deliver. -

Dietary Patterns, Foods and Nutrients, and Health Outcomes What Is The
Appendix E-2.27: Evidence Portfolio Appendix E2.27: Evidence Portfolio Part D. Chapter 2: Dietary Patterns, Foods and Nutrients, and Health Outcomes What is the relationship between dietary patterns and measures of body weight or obesity? Conclusion Statement: The DGAC concurs with the 2013 AHA/ACC/TOS Guideline for the Management of Overweight and Obesity1 that strong evidence demonstrates that, preferably as part of a comprehensive lifestyle intervention carried out by multidisciplinary teams of professionals or nutrition professionals, overweight and obese adults can achieve weight loss through a variety of dietary patterns that achieve an energy deficit. Clinically meaningful weight losses that were achieved ranged from 4 to 12 kg at 6-month follow-up. Thereafter, slow weight regain is observed, with total weight loss at 1 year of 4 to 10 kg and at 2 years of 3 to 4 kg. However, some dietary patterns may be more beneficial in the long-term for cardiometabolic health. DGAC Grade: Strong The DGAC concurs with the NEL Dietary Patterns Systematic Review Project2 that moderate evidence indicates dietary patterns that are higher in vegetables, fruits, and whole grains; include seafood and legumes; are moderate in dairy products (particularly low and non-fat dairy) and alcohol; lower in meats (including red and processed meats), and low in sugar-sweetened foods and beverages, and refined grains are associated with favorable outcomes related to healthy body weight (including lower BMI, waist circumference, or percent body fat) or risk of obesity. Components of the dietary patterns associated with these favorable outcomes include higher intakes of unsaturated fats and lower intakes of saturated fats, cholesterol, and sodium. -

Glycemic Index
GLYCEMIC INDEX High GI Foods = GI of 70+ (Try to avoid. Keep as a reward.) Medium GI = GI of 55 to 69. (Use caution. Avoid when possible.) Low GI = GI of 0 to 54. (This is your target zone. Choose foods FRUITS Cherries 22 Grapefruit 25 Prunes 29 Apricots, dried 30 Apple 38 Peach, canned in juice 38 Pear, fresh 38 Plum 39 Strawberries 40 Orange, Navel 42 Peach, fresh 42 Pear, canned 43 Grapes 46 Mango 51 Banana 52 Fruit Cocktail 55 Papaya 56 Raisins 56 Apricots, fresh 57 Kiwi 58 Figs, dried 61 Apricots, canned 64 Cantaloupe 65 Pineapple, fresh 66 Watermelon 72 Dates 103 Food GI Value GRAINS & RICE Barley, pearled 25 Converted, White 38 Reference: http://www.glycemicedge.com/glycemic-index-chart/ Long grain, White 44 Buckwheat 54 Brown 55 Basmati 58 Couscous 65 Cornmeal 68 Aborio 69 Short grain, White 72 Instant, White 87 Wild rice 87 Glutinous (Sticky) 98 BEANS & PEAS GI Value Chana Dal 8 Chickpeas, dried 28 Kidney Beans, dried 28 Lentils 29 Lima Beans (frozen) 32 Yellow Split Peas 32 Chickpeas, canned 42 Blackeyed Peas, canned 42 Baked Beans 48 Kidney Beans, canned 52 VEGETABLES Broccoli 10 Cabbage 10 Lettuce 10 Mushrooms 10 Onions 10 Red Peppers 10 Carrots 49 Green peas 48 Corn, fresh 60 Beets 64 Pumpkin 75 Parsnips 97 DAIRY Yogurt, artificially sweetened 14 Whole milk 31 Skim milk 32 Yogurt, sweetened 33 Ice cream, premium 38 Reference: http://www.glycemicedge.com/glycemic-index-chart/ Ice cream, low fat 43 SWEETNER Glucose 96 Fructose 22 Lactose 46 Sucrose (white sugar) 64 Brown sugar 64 Barley malt syrup 42 Brown rice syrup 25 Raw honey 30 Agave syrup 15 High fructose corn syrup 62 Stevia less than 1 Sugar cane juice 43 Evaporated cane juice 55 Maple syrup 54 Black strap molasses 55 The materials and content contained on this form are for general holistic nutrition information only to help support and enhance the body’s own healing properties and are not intended to be a substitute for professional medical advice, diagnosis or treatment for any medical condition. -

A Review of the Relationship Between Dietary Glycemic Index and Glycemic Load and Type 2 Diabetes
eISSN: 2476-7425 pISSN: 2476-7417 JNFS 2016; 1 (1): 73-79 Website: jnfs.ssu.ac.ir A Review of the Relationship between Dietary Glycemic Index and Glycemic Load and Type 2 Diabetes 1,2 3 4 Fahime Zeinali; MSc , Hadis Asadi Samani; BSc , Omid Toupchian; PhD , Shima Abdollahi; MSc1,2 & Mehnoosh Samadi; MSc*3 1 Nutrition and Food Security Research Center, Shahid Sadoughi University of Medical Sciences, Yazd, Iran. 2 Department of Nutrition, School of Public Health, Shahid Sadoughi University of Medical Sciences, Yazd, Iran. 3 Student Research Committee, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran 4 Department of Cellular and Molecular Nutrition, School of Nutritional Sciences and Dietetics, Tehran University of Medical Sciences, Tehran, Iran. ARTICLE INFO ABSTRACT REVIEW ARTICLE Background: In recent decades, studies on type 2 diabetes (T2D), have adopted a new approach to the field of a more complete collection of variables Article history: related to the lifestyles and diet of people. Diet is an important factor in Received: 9 Apr 2016 increasing the rate of T2D among individuals. Considering the consumption of Revised: 5 Jun 2016 a high-carbohydrate diet, little attention has been paid to the type of Accepted: 24 Jul 2016 carbohydrates consumed in the incidence of T2D. The present study aimed to review the literature on the relationship between the glycemic index (GI), the glycemic load, (GL) and T2D, it also targets at evaluating and comparing the *Corresponding author: results of similar studies in other countries Methods: Using search engines, Downloaded from jnfs.ssu.ac.ir at 11:48 IRST on Saturday October 2nd 2021 Student Research Committee, including PubMed, Science Direct, Embase and Scopus, and key words such Ahvaz Jundishapur University as GI, GL, diabetes; articles with cross-sectional, clinical trial, Prospective and of Medical Sciences, Ahvaz, retrospective cohort designs between 2000 to 2016 were selected. -
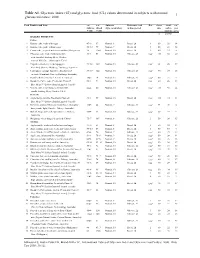
And Glycemic Load (GL) Values Determined in Subjects with Normal 1 Glucose Tolerance: 2008
Table A1. Glycemic index (GI) and glycemic load (GL) values determined in subjects with normal 1 glucose tolerance: 2008 Food Number and Item GI2 GI2 Subjects Reference food Ref. Serve Avail. GL3 (Glucose (Bread (type & number) & time period Size carbo- per = 100) = 100) hydrate serve g g/serve BAKERY PRODUCTS Cakes 1 Banana cake, made with sugar 47±8 67 Normal, 8 Bread, 2h 1 60 29 14 2 Banana cake, made without sugar 55±10 79 Normal, 7 Bread, 2h 1 60 22 12 3 Carrot cake, prepared with coconut flour (Philippines) 36 52±3 Normal, 10 Bread, 2h 2 60 23 8 4 Chocolate cake made from packet mix 38±3 54 Normal, 10 Glucose, 2h UO4 111 52 20 with chocolate frosting (Betty Crocker, General Mills Inc., Minneapolis, USA) 5 Cupcake, strawberry-iced (Squiggles, 73±12 104 Normal, 10 Glucose, 2h UO4 38 26 19 Farmland, Grocery Holdings, Tooronga, Australia) 6 Lamingtons (sponge dipped in chocolate and 87±17 124 Normal, 10 Glucose, 2h UO4 50 29 25 coconut) (Farmland, Grocery Holdings, Australia) 7 Pound cake 0% (Bimbo S.A de C.V, Mexico) 38±5 54 Normal, 12 Glucose, 2h UO4 60 25 9 8 Raspberry Coffee cake, President's Choice® 50±4 71 Normal, 10 Bread, 2h UO5 60 26 13 Blue Menu™ (Loblaw Brands Limited, Canada) 9 Vanilla cake, made from packet mix with 42±4 60 Normal, 10 Glucose, 2h UO4 111 58 24 vanilla frosting (Betty Crocker, USA) Desserts 10 Apple Berry crumble, President's Choice® 41±3 59 Normal, 10 Bread, 2h UO5 165 34 14 Blue Menu™ (Loblaw Brands Limited, Canada) 11 Bavarian (mousse filling on biscuit base), Chocolate 31±5 44 Normal, 9 Glucose, 2h UO6 -

Glycemic Index & Glycemic Load
Glycemic Index & Glycemic Load What is the glycemic index? The glycemic index of a food refers to the effect the food has on the body’s blood sugar levels. Blood sugar levels are raised after you eat foods containing carbohydrates (sugars and starches). Various carbohydrate-containing foods affect blood sugar levels differently. The glycemic index compares the rise in blood sugar level after eating a particular food to a reference food, often the sugar, glucose. (Glucose is a very basic sugar and not the same as table sugar). One of the foods that is often used as an How can I use the glycemic index to example is white bread. It has a relatively high improve my health? glycemic index of 70, when compared to By making careful food choices, you can glucose, which has an index of 100. influence your hunger and energy as well as blood sugar levels, cholesterol and triglyceride levels. If you have problems controlling how much food you eat, have hypoglycemia, diabetes, or high triglyceride cholesterol levels, paying attention to the glycemic index in your food choices will be helpful. What are some guidelines to follow in using the glycemic index? If you think that considering glycemic index in your diet would be helpful, follow these guidelines. 1. Eat low and medium glycemic index foods like beans, oatmeal, and whole grain pasta Why is the glycemic index important? regularly but in moderate quantity. Eat high The higher the rise in glucose in the blood glycemic index foods like bread, bagels, stream, the more insulin is produced to store it. -

Editorial Dietary Fibre and Diabetes Revisited
European Journal of Clinical Nutrition (2001) 55, 919–921 ß 2001 Nature Publishing Group All rights reserved 0954–3007/01 $15.00 www.nature.com/ejcn Editorial Dietary fibre and diabetes revisited J Mann1 1Deparment of Human Nutrition, University of Otago, New Zealand Dietary guidelines for people with diabetes appear to drate – high fibre diet was associated with a dramatic change from time to time and advice from various appar- improvement in glycaemic control and reduction in insulin ently authoritative sources differs — to the extent that those dose (Kiehm et al, 1976). In fact such observations were who have not carefully followed the reasoning behind the not entirely new, similar observations having been made recommendations might be forgiven for being somewhat many years earlier by Kempner using his rice diet (Kemp- dismissive regarding the extent to which dietary treatment ner et al, 1958). Inspired by the work of Anderson and also can influence glycaemic control and risk factor manage- by the studies of Stone and Connor (1963), who drew ment. The recently revised ‘Recommendations for the attention to the potential of the low carbohydrate – high fat nutritional management of patients with diabetes mellitus’ diet, then in widespread use, to increase cardiovascular prepared by the Diabetes and Nutrition Study Group risk, our own group in Oxford, England embarked on a (DNSG) of the European Association for the Study of series of carefully controlled studies which helped to Diabetes (EASD) help to place in perspective apparent further understand the role of dietary fibre (Simpson discrepancies between advice emanating from groups of et al, 1979a; Simpson et al, 1979b; Simpson et al, 1981; researchers or expert committees (Diabetes and Nutrition Simpson et al, 1982; Lousley et al, 1984; Geekie et al, Study Group, 2000).