Pathology and Molecular Mechanisms of Coarctation of the Aorta and Its Association with the Ductus Arteriosus
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
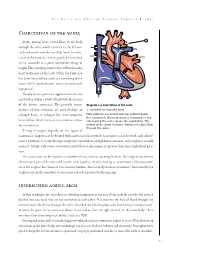
Coarctation of the Aorta Interrupted Aortic Arch
T HE P EDIATRIC C ARDIAC S URGERY I NQUEST R EPORT Coarctation of the aorta In the normal heart, blood flows to the body through the aorta, which connects to the left ven- tricle and arches over the top of the heart.In coarc- tation of the aorta,the aorta is pinched (in medical terms, coarcted) at a point somewhere along its length. This pinching restricts blood flow from the heart to the rest of the body. Often, the baby also has other heart defects, such as a narrowing of the aortic arch (in medical terms,transverse aortic arch hypoplasia). Usually no symptoms are apparent at birth, but can develop within a week of birth with the closure of the ductus arteriosus. The possible conse- Diagram 2.9 Coarctation of the aorta quences of this condition are poor feeding, an 1 – pinched or coarcted aorta enlarged heart, an enlarged liver and congestive Flow patterns are normal but are reduced below the coarctation. Blood pressure is increased in ves- heart failure. Blood pressure also increases above sels leaving the aorta above the coarctation. The the constriction. broken white arrow indicates diminished blood flow through the aorta. Timing of surgery depends on the degree of coarctation. Surgery may be delayed with mild coarctation (which sometimes is not detected until adoles- cence). However, if a baby develops congestive heart failure or high blood pressure, early surgery is usually required. A baby with severe coarctation should have early surgery to prevent long-term high blood pres- sure. The coarctation can be repaired in a number of ways without opening the heart.The surgeon can remove the narrowed part of the aorta and sew the ends together, thereby creating an anastomosis. -

Thoracic Aorta
GUIDELINES AND STANDARDS Multimodality Imaging of Diseases of the Thoracic Aorta in Adults: From the American Society of Echocardiography and the European Association of Cardiovascular Imaging Endorsed by the Society of Cardiovascular Computed Tomography and Society for Cardiovascular Magnetic Resonance Steven A. Goldstein, MD, Co-Chair, Arturo Evangelista, MD, FESC, Co-Chair, Suhny Abbara, MD, Andrew Arai, MD, Federico M. Asch, MD, FASE, Luigi P. Badano, MD, PhD, FESC, Michael A. Bolen, MD, Heidi M. Connolly, MD, Hug Cuellar-Calabria, MD, Martin Czerny, MD, Richard B. Devereux, MD, Raimund A. Erbel, MD, FASE, FESC, Rossella Fattori, MD, Eric M. Isselbacher, MD, Joseph M. Lindsay, MD, Marti McCulloch, MBA, RDCS, FASE, Hector I. Michelena, MD, FASE, Christoph A. Nienaber, MD, FESC, Jae K. Oh, MD, FASE, Mauro Pepi, MD, FESC, Allen J. Taylor, MD, Jonathan W. Weinsaft, MD, Jose Luis Zamorano, MD, FESC, FASE, Contributing Editors: Harry Dietz, MD, Kim Eagle, MD, John Elefteriades, MD, Guillaume Jondeau, MD, PhD, FESC, Herve Rousseau, MD, PhD, and Marc Schepens, MD, Washington, District of Columbia; Barcelona and Madrid, Spain; Dallas and Houston, Texas; Bethesda and Baltimore, Maryland; Padua, Pesaro, and Milan, Italy; Cleveland, Ohio; Rochester, Minnesota; Zurich, Switzerland; New York, New York; Essen and Rostock, Germany; Boston, Massachusetts; Ann Arbor, Michigan; New Haven, Connecticut; Paris and Toulouse, France; and Brugge, Belgium (J Am Soc Echocardiogr 2015;28:119-82.) TABLE OF CONTENTS Preamble 121 B. How to Measure the Aorta 124 I. Anatomy and Physiology of the Aorta 121 1. Interface, Definitions, and Timing of Aortic Measure- A. The Normal Aorta and Reference Values 121 ments 124 1. -

Atypical Coarctation of Aorta
Atypical coarctation of aorta Authors: Professor Harald Kaemmerer1 and Doctor Alfred Hager Creation Date: January 2003 Scientific Editor: Professor Marie-Christine Seghaye 1Department of pediatric cardiology, Deutsches Herzzentrum München, Lazarettstr. 36, 80636 München, Germany. [email protected] Abstract Keywords Disease name and synonyms Definition Frequency Clinical presentation Etiology Diagnostic methods Treatment References Abstract Atypical coarctation of aorta (CoA) is characterized by localized or extended narrowing of the ascending aorta, of the descending thoracic aorta at the level of the diaphragm, or of the abdominal aorta. Symptoms are attributed to upper body arterial hypertension and may include headache, abdominal angina, leg fatigue at exercise, cold feet and intermittent claudication. A pressure difference between arms and legs may exist as it is the case for the isthmic CoA. A systolic vascular murmur can be heard over the region of stenosis. This disease is often caused by an arteritis (Takayasu arteritis or aortitis) or a fibromuscular dysplasia. Some forms may be congenital. It can be associated with neurofibromatosis such as von Recklinghausen disease or Williams syndrome. This rare condition affects 0.5% to 2% of individuals with CoA. The symptomatic treatment consists of antihypertensive medication. The causal treatment is either percutaneous transluminal angioplasty with or without endovascular stent placement or surgery. Keywords Coarctation, aortic isthmus, atypical, ectopic, Takayasu arteritis, neurofibromatosis (Recklinghausen disease), Williams syndrome, rubella syndrome, tuberous sclerosis, Alagille syndrome. Disease name and synonyms Atypical CoA is characterized by a localized or • Atypical coarctation of the aorta extended narrowing of the ascending aorta, of • Coarctation of the abdominal aorta the descending thoracic aorta at the level of the • Middle aortic syndrome diaphragm or of the abdominal aorta. -

Coarctation of the Aorta: from Fetal Life to Adulthood
Cardiology Journal 2011, Vol. 18, No. 5, pp. 487–495 10.5603/CJ.2011.0003 Copyright © 2011 Via Medica REVIEW ARTICLE ISSN 1897–5593 Coarctation of the aorta: From fetal life to adulthood Damien Kenny, Ziyad M. Hijazi Rush Center for Congenital and Structural Heart Disease, Rush University Medical Center, Chicago, IL, USA Abstract Coarctation of the aorta was once viewed as a simple discrete narrowing of the aortic isthmus that could be ‘cured’ by surgical intervention. It is now clear that this condition may: (1) affect the aortic arch in a highly variable manner; (2) be associated with a host of other left sided heart lesions; (3) represent a wider vasculopathy within the pre-coarctation arterial tree, leading to significant prevalence of hypertension by adolescence, and subsequent risk of early morbidity and death. This review outlines the evaluation and treatment of this disease from pre-natal to adult life. (Cardiol J 2011; 18, 5: 487–495) Key words: surgery, echocardiography, stenting, hypertension, vasculopathy Introduction Coarctation of the aorta (CoA) is the fifth most common congenital heart defect, accounting for 6–8% of live births with congenital heart disease, with an estimated incidence of 1 in 2,500 births [1–3]. It is likely that the incidence is higher in stillborn babies [4]. It affects more male babies than female, with a reported ratio in males of between 1.27:1 and 1.74:1 [5, 6]. It usually manifests as a discrete con- striction of the aortic isthmus (Fig. 1). However, it is more likely to represent a spectrum of aortic nar- rowing from this discrete entity to tubular hypopla- sia, with many variations seen in between these two extremes. -

The Aorta, and Pulmonary Blood Flow
Br Heart J: first published as 10.1136/hrt.36.5.492 on 1 May 1974. Downloaded from British HeartJournal, I974, 36, 492-498. Relation between fetal flow patterns, coarctation of the aorta, and pulmonary blood flow Elliot A. Shinebourne and A. M. Elseed From the Department of Paediatrics, Brompton Hospital, National Heart and Chest Hospitals, London Intracardiac anomalies cause disturbances in fetal flow patterns which in turn influence dimensions of the great vessels. At birth the aortic isthmus, which receives 25 per cent of the combinedfetal ventricular output, is normally 25 to 30 per cent narrower than the descending aorta. A shelf-like indentation of the posterior aortic wall opposite the ductus characterizes thejunction of the isthmus with descending aorta. In tetralogy ofFallot, pulmonary atresia, and tricuspid atresia, when pulmonary blood flow is reduced from birth, the main pul- monary artery is decreased and ascending aorta increased in size. Conversely in intracardiac anomalies where blood is diverted away from the aorta to the pulmonary artery, isthmal narrowing or the posterior indentation may be exaggerated. Analysis of I62 patients with coarctation of the aorta showed 83 with an intracardiac anomaly resulting in increased pulmonary bloodflow and 21 with left-sided lesions present from birth. In contrast no patients with coarctation were found with diminished pulmonary flow or right-sided obstructive lesions. From this evidence the hypothesis is developed that coarctation is prevented whenflow in the main pulmonary artery is reduced in thefetus. http://heart.bmj.com/ The association of coarctation with left-sided establishing the complete intracardiac diagnosis in I62 obstructive lesions such as mitral and aortic stenosis patients with coarctation. -

Aortic Coarctation and Interrupeted
Aortic Coarctation and Interrupted Aortic Arch 25 Keila N. Lopez, Sebastian C. Tume, Stuart R. Hall, Aimee Liou, S. Kristen Sexson Tejtel, Carlos M. Mery Coarctation of the aorta (CoA), aortic arch hypoplasia, and interrupted aortic arch (IAA) are congenital conditions where a portion of the aorta (not including the aortic valve) measure small for BSA or a segment of the arch is atretic or missing. ese lesions can present in the newborn period, childhood, and more rarely, in the adult period. Timing of presentation is dependent on the severity of the lesion (degree and extent of arch narrowing) as well as the presence of a PDA and aortopulmonary collaterals. Arch lesions may also be accompanied by other left-sided obstructive lesions including an abnormal aortic valve, mitral valve, or LV. For multilevel left-heart hypoplasia, see Chapter . e aortic arch is usually divided into dierent segments (Figure -): • Proximal arch: between the innominate artery and left carotid artery • Distal arch: between the left carotid artery and the left subclavian artery • Isthmus: between the left subclavian artery and the ductus arteriosus or ligamen- tum arteriosum e dimensions of each of the aortic arch segments are important to dene the clin- ical presentation and management of patients with aortic arch hypoplasia. Focal CoA classically presents with signicant narrowing at the level of the isthmus. IAA is classied into types depending on the aortic arch segment involved with the interruption: • Type A: the interruption is distal to the left subclavian artery • Type B: the interruption is between the left common carotid artery and the left subclavian artery • Type C: the interruption is between the innominate artery and the left common carotid artery Pathophysiology and Clinical Presentation Fetal Mild aortic arch lesions are often dicult to detect in utero, and may only be detected postnatally, after the PDA closes. -

Repaired Coarctation of the Aorta, Persistent Arterial Hypertension and the Selfish Brain Jonathan C
Rodrigues et al. Journal of Cardiovascular Magnetic Resonance (2019) 21:68 https://doi.org/10.1186/s12968-019-0578-8 RESEARCH Open Access Repaired coarctation of the aorta, persistent arterial hypertension and the selfish brain Jonathan C. L. Rodrigues1,2,3*, Matthew F. R. Jaring4, Melissa C. Werndle4, Konstantina Mitrousi2, Stephen M. Lyen4, Angus K. Nightingale5, Mark C. K. Hamilton4, Stephanie L. Curtis6, Nathan E. Manghat4, Julian F. R. Paton2,5,7† and Emma C. Hart2,5*† Abstract Background: It has been estimated that 20–30% of repaired aortic coarctation (CoA) patients develop hypertension, with significant cardiovascular morbidity and mortality. Vertebral artery hypoplasia (VAH) with an incomplete posterior circle of Willis (ipCoW; VAH + ipCoW) is associated with increased cerebrovascular resistance before the onset of increased sympathetic nerve activity in borderline hypertensive humans, suggesting brainstem hypoperfusion may evoke hypertension to maintain cerebral blood flow: the “selfish brain” hypothesis. We now assess the “selfish brain” in hypertension post-CoA repair. Methods: Time-of-flight cardiovascular magnetic resonance angiography from 127 repaired CoA patients (34 ± 14 years, 61% male, systolic blood pressure (SBP) 138 ± 19 mmHg, diastolic blood pressure (DBP) 76 ± 11 mmHg) was compared with 33 normotensive controls (42 ± 14 years, 48% male, SBP 124 ± 10 mmHg, DBP 76 ± 8 mmHg). VAH was defined as < 2 mm and ipCoW as hypoplasia of one or both posterior communicating arteries. Results: VAH + ipCoW was more prevalent in repaired CoA than controls (odds ratio: 5.8 [1.6–20.8], p = 0.007), after controlling for age, sex and body mass index (BMI). VAH + ipCoW was an independent predictor of hypertension (odds ratio: 2.5 [1.2–5.2], p = 0.017), after controlling for age, gender and BMI. -

Bicuspid Aortic Valve and Aortic Coarctation in Congenital Heart Disease—Important Aspects for Treatment with Focus on Aortic Vasculopathy
788 Mini-Review Bicuspid aortic valve and aortic coarctation in congenital heart disease—important aspects for treatment with focus on aortic vasculopathy Christoph Sinning1,2#, Elvin Zengin1#, Rainer Kozlik-Feldmann3, Stefan Blankenberg1,2, Carsten Rickers3, Yskert von Kodolitsch1, Evaldas Girdauskas4 1Department of General and Interventional Cardiology, University Heart Center Hamburg, Hamburg, Germany; 2German Center of Cardiovascular Research (DZHK), Partner Site Hamburg/Kiel/Lübeck, Germany; 3Department of Pediatric Cardiology, 4Department of Cardiac and Cardiovascular Surgery, University Heart Center Hamburg, Hamburg, Germany Contributions: (I) Conception and design: C Sinning, E Zengin, Y von Kodolitsch, E Girdauskas; (II) Administrative support: None; (III) Provision of study materials or patients: None; (IV) Collection and assembly of data: C Sinning, E Zengin; (V) Data analysis and interpretation: C Sinning, E Zengin, Y von Kodolitsch, E Girdauskas; (VI) Manuscript writing: All authors; (VII) Final approval of manuscript: All authors. #These authors contributed equally to this work. Correspondence to: Christoph Sinning, MD. Department of General and Interventional Cardiology, Universtiy Heart Center Hamburg, Martinistr. 52, 20246 Hamburg, Germany. Email: [email protected]. Abstract: Prevalence of congenital heart disease (CHD) is constantly increasing during the last decades in line with the treatment options for patients ranging from the surgical as well to the interventional spectrum. This mini-review addresses two of the most common defects with bicuspid aortic valve (BAV) and coarctation of the aorta (CoA). Both diseases are connected to aortic vasculopathy which is one of the most common reasons for morbidity and mortality in young patients with CHD. The review will focus as well on other aspects like medication and treatment of pregnant patients with BAV and CoA. -

Coarctation of the Aorta What Is Coarctation of the Aorta? Mal Mitral Valve, and Coarctation
HEART MATT ERS A PUBLICATION OF THE ADULT CONGENITAL HEART ASSOCIATION ACHA Q and A: Coarctation of the Aorta What is coarctation of the aorta? mal mitral valve, and coarctation. Your family members may People with coarctation of the aorta are born with an aorta want to get tested for heart defects. This usually involves an that is too narrow . The aorta is the longest blood vessel in the echocardiogram, which is an ultrasound picture of the heart . body . Blood is pumped from the left ventricle into the aorta. What other defects are common in coarctation? It then branches out into the right arm, head , and left arm . In 50-85% of coarctation patients also have a bicuspid aortic coarctation the aorta can be narrow in different places and in valve. This means the aortic valve has two flaps instead of different ways. Some people have a short narrow section. three. Some people with COA are also born with a hole in the Others have long areas of their aorta that are too narrow. If lower wall of the heart (ventricular septal defect). A smaller the aorta is very narrow, blood will not be able to pass. This number have problems with other heart valves such as the kind of coarctation is usually found at birth. If the narrowing mitral valve. People with coarctation are also at risk from is less severe, it may not be detected until later in life . swelling in their blood vessels (aneurysms). The causes and How common is coarctation? risks of aneurysms are discussed below. -

Moyamoya Disease and Aortic Coarctation in a Patient With
Case Report Olgu Sunumu 85 Moyamoya disease and aortic coarctation in a patient with common brachiocephalic trunk Moyamoya hastal›¤› ve aort koarktasyonunun efllik etti¤i bir brakiyosefalik kütük olgusu Kadir Babao¤lu, Tevfik Demir*, Levent Salt›k*, fieyhan Kutlu¤**, Civan Ifllak*** Department of Pediatric Cardiology, Kocaeli University, Faculty of Medicine, Kocaeli, Turkey Departments of *Pediatric Cardiology, **Pediatrics and ***Radiology, Cerrahpafla Faculty of Medicine, Istanbul University, Istanbul, Turkey Introduction yond which severe stenosis of the both carotid arteries were seen. The network of collateral vessels was demonstrated at the dien- Moyamoya disease is characterized by a slowly progressive cephalic region (Fig. 2). A child had been treated with anticonvul- stenosis and obliteration of the large vessels at the base of the sant and subsequently had been advised to have physiotherapy. At brain, affecting mainly the supraclinoid segment of the internal ca- the last control, upper limb hypertension was established. The rotid artery and the initial portion of the anterior or middle cereb- systolic pressure gradient between the right upper and lower limbs ral arteries and the posterior cerebral arteries (1). Due to the slow was 30 mmHg. Echocardiography revealed a high systolic gradient progression of the disease and in response to progressive cereb- (45 mmHg) at the aortic isthmus. Cardiac catheterization and angi- ral ischemia, a large network of collateral vessels is formed from ography revealed CBT, coarctation of the aorta and stenosis at the the external carotid arteries, the vertebra-basilar system and ot- origin of left subclavian artery (Fig. 3). A cerebral angiography was her vessels (2). done at the same time and confirmed the diagnosis of Moyamoya The idiopathic or primary form of Moyamoya disease, which is disease. -

Hypoplastic Aortic Arch, Coarctation of the Aorta and Poststenotic Aneurysm
272 Türk Kardiyol Dern Arş - Arch Turk Soc Cardiol 2015;43(3):272-274 doi: 10.5543/tkda.2015.89084 A rare combination of vascular anomalies: Hypoplastic aortic arch, coarctation of the aorta and poststenotic aneurysm Nadir bir damar anomalisi kombinasyonu: Hipoplastik aort yayı, aort koarktasyonu ve poststenotik anevrizma Nermin Bayar, M.D., Şakir Arslan, M.D., Çağın Mustafa Üreyen, M.D., Selçuk Küçükseymen, M.D., Bekir Erol, M.D.# Department of Cardiology, Antalya Training and Research Hospital, Antalya #Department of Radiology, Antalya Training and Research Hospital, Antalya Summary– Coarctation of the aorta is the fifth most com- Özet– Aort koarktasyonu erişkinlerde beşinci sıklıkta görü- mon congenital cardiac anomaly encountered in adults. It len doğumsal kalp anomalisidir. Prognoz için erken tanı ve is important for prognosis to diagnose and treat this anom- tedavi önemlidir. Aort koarktasyonu bulunan olgularda tu- aly early. An aneurysm might develop due to tunica media nika medya anormallikleri anevrizma gelişimine neden ola- abnormalities in patients with coarctation of the aorta. We bilmektedir. Bu yazıda, çıkan aort anevrizması, hipoplastik hereby present an adult case with a very rare combination aort yayı, aort koarktasyonu ve poststenotik anevrizmayı of vascular anomalies including ascending aorta aneurysm, içeren çok nadir damar anomalisi birlikteliği olan erişkin bir hypoplastic aortic arch, coarctation of the aorta and postste- hasta sunuldu. notic aneurysm. oarctation of the aorta constitutes 5-8% of all pressure was regulated. Abbreviations: congenital cardiac anomalies.[1] Moreover, bi- Physical examination re- C 3D Three-dimensional cuspid aortic valve and ventricular septal defect may vealed a II/IV diastolic CT Computed tomography accompany coarctation of the aorta.[2] An aortic aneu- murmur in the meso- TTE Transthoracic echocardiograhy rysm may also develop owing to medial abnormalities cardiac area. -

Coarctation of the Aorta P
Coarctation of the Aorta P. Syamasundar Rao, MD Address the media and superimposed neointimal tissue. The local- Division of Pediatric Cardiology, The University of Texas/Houston ized constriction may form a shelf-like structure with an Medical School, 6431 Fannin, MSB 3.130, Houston, TX 77030, USA. eccentric opening or it may be a membranous curtain-like E-mail: [email protected] structure with a central or eccentric opening. The coarcta- Current Cardiology Reports 2005, 7:425–434 tion may be discrete, or a long segment of the aorta may be Current Science Inc. ISSN 1523-3782 Copyright © 2005 by Current Science Inc. narrowed; the former is more common. In the past, AC has been described as preductal (or infantile) type and post- ductal (or adult) type, depending upon whether the coarc- Coarctation of the aorta is an important, treatable cause of tation segment is proximal or distal to the ductus secondary hypertension. Its prevalence varies from 5% to arteriosus, respectively. However, a detailed review of the 8% of all congenital heart defects. This condition is most anatomy suggests that all coarctations are juxtaductal. often detected because of a murmur or hypertension found Dilatation of the descending aorta immediately distal on routine examination. Delayed or absent femoral pulses to the coarctation segment, poststenotic dilatation, is usu- and an arm/leg systolic blood pressure difference of 20 mm ally present. Varying degrees of hypoplasia of the isthmus Hg or more in favor of the arms may be considered as evi- of the aorta (the portion of the aorta between the origin of dence for aortic coarctation.