Zuclopenthixol Versus Placebo for Schizophrenia (Review)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Standard Operating Procedure Initiation And
STANDARD OPERATING PROCEDURE Title: Procedure Document Version INITIATION AND PRESCRIBING OF PALIPERIDONE No: Replaced: LONG ACTING INJECTION (PLAI) Clinical N/A 2 SOP 03 Procedure Written By: Procedure Approved By: Approved Review Page Senior Pharmacist Gurj Bhella Date: Date: Deputy Chief Pharmacist Chief Pharmacist 22/05/2018 22/05/2020 1 of 3 Objective To ensure that all prescriptions for paliperidone are initiated by consultants on a named-patient basis and that a record of all patients is kept. Scope All BCPFT consultant teams, Pharmacy Team. Procedure 1. When a patient is identified as being a candidate for paliperidone LAI, the following criteria must be fulfilled: a. The prescribing of a typical antipsychotic depot injection first line has been tried to no effect or has not been tolerated, or is not considered clinically appropriate. b. PLAI to be initiated by Consultants only and be prescribed for its licensed indication only i.e. for maintenance treatment of schizophrenia in adult patients stabilised with risperidone (Oral or LAI) or have responded to it previously. In selected adult patients with schizophrenia and previous responsiveness to oral paliperidone or risperidone, it may be used without prior stabilisation with oral treatment if psychotic symptoms are mild to moderate and a long-acting injectable treatment is needed. Patients may now be switched from oral risperidone as well as risperidone LAI. c. PLAI must be prescribed in line with the treatment guidance for the use of long acting injections contained within NICE guideline CG178 ‘Psychosis and schizophrenia in adults: treatment and management’ issued February 2014. d. Paliperidone LAI must be prescribed once each calendar month i.e.12 times a year. -

PRODUCT INFORMATION Perphenazine Item No
PRODUCT INFORMATION Perphenazine Item No. 20735 CAS Registry No.: 58-39-9 HO Formal Name: 4-[3-(2-chloro-10H-phenothiazin-10-yl) N propyl]-1-piperazineethanol N Synonyms: NSC 150866, SCH 3940 MF: C21H26ClN3OS FW: 404.0 Purity: ≥98% N Cl UV/Vis.: λmax: 257, 312 nm Supplied as: A crystalline solid Storage: -20°C S Stability: ≥2 years Information represents the product specifications. Batch specific analytical results are provided on each certificate of analysis. Laboratory Procedures Perphenazine is supplied as a crystalline solid. A stock solution may be made by dissolving the perphenazine in the solvent of choice, which should be purged with an inert gas. Perphenazine is soluble in organic solvents such as ethanol, DMSO, and dimethyl formamide. The solubility of perphenazine in these solvents is approximately 5, 20, and 30 mg/ml, respectively. Description 1 Perphenazine is a typical antipsychotic. It binds to dopamine D2, α1A-, α2A-, α2B-, and α2C-adrenergic, M3 muscarinic, and histamine H1 receptors (Kis = 1.4, 10, 1,848, 104.9, 85.2, 810.5 and 8 nM, respectively), as well as the serotonin (5-HT) receptor subtypes 5-HT1A, 5-HT2A, 5-HT2C, 5-HT6, and 5-HT7 (Kis = 421, 5.6, 132, 17, and 23 nM, respectively). Perphenazine (1, 5, and 10 mg/kg) enhances morphine-induced analgesia in the tail-flick and hot plate tests in rats.2 It reduces cannibalism in female mice when administered at doses of 2 and 4 mg/kg.3 Formulations containing perphenazine have been used in the treatment of schizophrenia and psychosis. References 1. -

Appendix 13C: Clinical Evidence Study Characteristics Tables
APPENDIX 13C: CLINICAL EVIDENCE STUDY CHARACTERISTICS TABLES: PHARMACOLOGICAL INTERVENTIONS Abbreviations ............................................................................................................ 3 APPENDIX 13C (I): INCLUDED STUDIES FOR INITIAL TREATMENT WITH ANTIPSYCHOTIC MEDICATION .................................. 4 ARANGO2009 .................................................................................................................................. 4 BERGER2008 .................................................................................................................................... 6 LIEBERMAN2003 ............................................................................................................................ 8 MCEVOY2007 ................................................................................................................................ 10 ROBINSON2006 ............................................................................................................................. 12 SCHOOLER2005 ............................................................................................................................ 14 SIKICH2008 .................................................................................................................................... 16 SWADI2010..................................................................................................................................... 19 VANBRUGGEN2003 .................................................................................................................... -

Datasheet Inhibitors / Agonists / Screening Libraries a DRUG SCREENING EXPERT
Datasheet Inhibitors / Agonists / Screening Libraries A DRUG SCREENING EXPERT Product Name : Aripiprazole Lauroxil Catalog Number : T14319 CAS Number : 1259305-29-7 Molecular Formula : C36H51Cl2N3O4 Molecular Weight : 660.71 Description: Aripiprazole lauroxil is cleaved by body’s enzyme esterase to N-hydroxymethyl aripiprazole (plus lauric acid) and then to aripiprazole (plus formaldehyde), no toxicity[1]. Aripiprazole lauroxil, an N-acyloxymethyl prodrug of aripiprazole, is a Long-acting injectable (LAI) typical antipsychotic for schizophrenia. Storage: 2 years -80°C in solvent; 3 years -20°C powder; Receptor (IC50) Others In vivo Activity Aripiprazole lauroxil (intravenous administration; 1.87 mg/ml) bioconversion in vivo involves the formation of an intermediate, N- hydroxymethyl aripiprazole. The in vitro data indicates a high bioconversion of aripiprazole lauroxil, thus, the concentration of N- hydroxymethyl aripiprazole observed in the animals dosed with aripiprazole lauroxil is surprisingly high[1]. Reference 1. Jann MW, et al. Long-Acting Injectable Second-Generation Antipsychotics: An Update and Comparison Between Agents.CNS Drugs. 2018 Mar;32(3):241-257. 2. Rohde M, et al. Biological conversion of aripiprazole lauroxil - An N-acyloxymethyl aripiprazole prodrug.Results Pharma Sci. 2014 May 2;4:19-25. FOR RESEARCH PURPOSES ONLY. NOT FOR DIAGNOSTIC OR THERAPEUTIC USE. Information for product storage and handling is indicated on the product datasheet. Targetmol products are stable for long term under the recommended storage conditions. Our products may be shipped under different conditions as many of them are stable in the short-term at higher or even room temperatures. We ensure that the product is shipped under conditions that will maintain the quality of the reagents. -

Ecstasy and the Concomitant Use of Pharmaceuticals
J.Copeland, P. Dillon & M. Gascoigne Ecstasy and the Concomitant Use of Pharmaceuticals NDARC Technical Report No. 201 ECSTASY AND THE CONCOMITANT USE OF PHARMACEUTICALS Jan Copeland (PhD), Paul Dillon & Michael Gascoigne Technical Report Number 201 ISBN: 1 877027 92 8 ©National Drug and Alcohol Research Centre, University of New South Wales, Sydney, 2004 This work is copyright. You may download, display, print and reproduce this material in unaltered form only (retaining this notice) for your personal, non-commercial use or use within your organisation. All other rights are reserved. Requests and enquiries concerning reproduction and rights should be addressed to the information manager, National Drug and Alcohol Research Centre, University of New South Wales, Sydney, NSW 2052, Australia. 2 ACKNOWLEDGEMENT This study and report was funded by the Australian Government Department of Health and Ageing’s National Illicit Drug Strategy. It was conducted concurrently with a study of aspects of information seeking among ecstasy users and there is some replication of the data presented on demographic characteristics and perception of ecstasy related harms in this Technical Report and Technical Report Number 202. 3 TABLE OF CONTENTS ACKNOWLEDGEMENT 3 TABLE OF CONTENTS 4 TABLE OF TABLES 5 EXECUTIVE SUMMARY 6 INTRODUCTION 7 Anti-depressant medication 8 5-hydroxytryptophan 9 Attention Deficit Hyperactivity Disorder 11 Sildenafil Citrate 12 Benzodiazepines 13 Study Ains 14 METHOD 15 Participants 15 Procedure 15 Measures 15 RESULTS 17 Demographics -

Clopixol Acuphase®)
Guidelines for the use of zuclopenthixol acetate injection (Clopixol Acuphase®) Version 3.1 – January 2018 GUIDELINE REPLACED Version 3 RATIFYING COMMITTEE Drugs and Therapeutics Group DATE RATIFIED October 2015 NEXT REVIEW DATE January 2021 EXECUTIVE SPONSOR Executive Medical Director ORIGINAL AUTHOR Jed Hewitt – Chief Pharmacist Review / update – 2015 Helen Manuell – Lead Pharmacist (ESx) Review / update - 2018 Jed Hewitt – Chief Pharmacist If you require this document in an alternative format, i.e. easy read, large text, audio or Braille please contact the pharmacy team on 01243 623349. Guidelines for the use of zuclopenthixol acetate injection (Clopixol Acuphase®) Introduction In the past, Acuphase® has often been too widely and possibly inappropriately used, sometimes without full regard being given to the fact that it is a potentially hazardous and toxic preparation with very little published information to support its use. Indeed, the Cochrane Library concludes that there is inadequate data on Acuphase® and no convincing evidence to support its use in acute psychiatric emergency. So far as possible, it should therefore be reserved for the minority of patients who have a prior history of previous use and good response, with use defined in an advance directive. Similarly, as a general rule Acuphase® should not be used for rapid tranquillisation unless such practice is also in accordance with an advance directive. Normally, Acuphase® should never be considered as a first-line drug for rapid tranquillisation as its onset of action will often not be rapid enough in these circumstances. In addition, the administration of an oil-based injection carries very high risk in a highly agitated patient. -

Product Data Sheet
Product data sheet MedKoo Cat#: 326728 Name: Aripiprazole lauroxil CAS#: 1259305-29-7 (lauroxil) Chemical Formula: C36H51Cl2N3O4 Exact Mass: 659.3257 Molecular Weight: 660.721 Product supplied as: Powder Purity (by HPLC): ≥ 98% Shipping conditions Ambient temperature Storage conditions: Powder: -20°C 3 years; 4°C 2 years. In solvent: -80°C 3 months; -20°C 2 weeks. 1. Product description: Aripiprazole lauroxil, aslo known as RDC 3317, is a long-acting injectable atypical antipsychotic. It is an N-acyloxymethyl prodrug of aripiprazole that is administered via intramuscular injection once every four to six weeks for the treatment of schizophrenia. Aripiprazole lauroxil was approved by the U.S. FDA on 5 October 2015. 2. CoA, QC data, SDS, and handling instruction SDS and handling instruction, CoA with copies of QC data (NMR, HPLC and MS analytical spectra) can be downloaded from the product web page under “QC And Documents” section. Note: copies of analytical spectra may not be available if the product is being supplied by MedKoo partners. Whether the product was made by MedKoo or provided by its partners, the quality is 100% guaranteed. 3. Solubility data Solvent Max Conc. mg/mL Max Conc. mM DMSO 8.33 12.61 4. Stock solution preparation table: Concentration / Solvent Volume / Mass 1 mg 5 mg 10 mg 1 mM 1.51 mL 7.57 mL 15.13 mL 5 mM 0.30 mL 1.51 mL 3.03 mL 10 mM 0.15 mL 0.76 mL 1.51 mL 50 mM 0.03 mL 0.15 mL 0.30 mL 5. -

Drug Information Sheet
University Health System PSYCHIATRIC SERVICES Antipsychotics "Depot" Injectable Typical Neuroleptics Fluphenazine Decanoate (Prolixin Decanoate®) Haloperidol Decanoate (Haldol Decanoate®) Course of Treatment: _________________________________________________________________________________ PURPOSE AND GENERAL INFORMATION 1. This medication is used to treat a variety of psychiatric problems such as overactivity, preoccupation with troublesome and recurring thoughts, and unpleasant and unusual experiences such as hearing and seeing things not normally heard nor seen. This medication will reduce or stop these experiences and help you remain outside the hospital. 2. This medication cannot "cure" the illness, but it can take away many of the symptoms or make them milder. It is important to take this medication as directed, even when you begin to feel better. It is necessary to continue taking this medication in order to keep feeling well. 3. Depot medication is an alternative to taking medicine by mouth. An injection is used to deposit medication into muscle tissue and from there the medication is slowly released into your system over a number of weeks. When medication is taken by mouth, it is quickly absorbed through the stomach and intestines; unfortunately, much of it is lost in this digestive process. 4. This medication does not produce euphoria (a high feeling) and is not addictive. BENEFITS Relief of Symptoms 1. Reduction or elimination of voices or visions not heard nor seen by others. 2. Reduction or elimination of frightening or strange beliefs and ideas not shared by others. 3. Decreased tension and agitation with more calm, relaxed feelings. 4. Improved concentration and clearer thinking; better control over thoughts and feelings with less hostile, strange, or aggressive thoughts. -

CENTRAL NERVOUS SYSTEM DEPRESSANTS Opioid Pain Relievers Anxiolytics (Also Belong to Psychiatric Medication Category) • Codeine (In 222® Tablets, Tylenol® No
CENTRAL NERVOUS SYSTEM DEPRESSANTS Opioid Pain Relievers Anxiolytics (also belong to psychiatric medication category) • codeine (in 222® Tablets, Tylenol® No. 1/2/3/4, Fiorinal® C, Benzodiazepines Codeine Contin, etc.) • heroin • alprazolam (Xanax®) • hydrocodone (Hycodan®, etc.) • chlordiazepoxide (Librium®) • hydromorphone (Dilaudid®) • clonazepam (Rivotril®) • methadone • diazepam (Valium®) • morphine (MS Contin®, M-Eslon®, Kadian®, Statex®, etc.) • flurazepam (Dalmane®) • oxycodone (in Oxycocet®, Percocet®, Percodan®, OxyContin®, etc.) • lorazepam (Ativan®) • pentazocine (Talwin®) • nitrazepam (Mogadon®) • oxazepam ( Serax®) Alcohol • temazepam (Restoril®) Inhalants Barbiturates • gases (e.g. nitrous oxide, “laughing gas”, chloroform, halothane, • butalbital (in Fiorinal®) ether) • secobarbital (Seconal®) • volatile solvents (benzene, toluene, xylene, acetone, naptha and hexane) Buspirone (Buspar®) • nitrites (amyl nitrite, butyl nitrite and cyclohexyl nitrite – also known as “poppers”) Non-Benzodiazepine Hypnotics (also belong to psychiatric medication category) • chloral hydrate • zopiclone (Imovane®) Other • GHB (gamma-hydroxybutyrate) • Rohypnol (flunitrazepam) CENTRAL NERVOUS SYSTEM STIMULANTS Amphetamines Caffeine • dextroamphetamine (Dexadrine®) Methelynedioxyamphetamine (MDA) • methamphetamine (“Crystal meth”) (also has hallucinogenic actions) • methylphenidate (Biphentin®, Concerta®, Ritalin®) • mixed amphetamine salts (Adderall XR®) 3,4-Methelynedioxymethamphetamine (MDMA, Ecstasy) (also has hallucinogenic actions) Cocaine/Crack -

Review of Pharmacokinetics and Pharmacogenetics in Atypical Long-Acting Injectable Antipsychotics
pharmaceutics Review Review of Pharmacokinetics and Pharmacogenetics in Atypical Long-Acting Injectable Antipsychotics Francisco José Toja-Camba 1,2,† , Nerea Gesto-Antelo 3,†, Olalla Maroñas 3,†, Eduardo Echarri Arrieta 4, Irene Zarra-Ferro 2,4, Miguel González-Barcia 2,4 , Enrique Bandín-Vilar 2,4 , Victor Mangas Sanjuan 2,5,6 , Fernando Facal 7,8 , Manuel Arrojo Romero 7, Angel Carracedo 3,9,10,* , Cristina Mondelo-García 2,4,* and Anxo Fernández-Ferreiro 2,4,* 1 Pharmacy Department, University Clinical Hospital of Ourense (SERGAS), Ramón Puga 52, 32005 Ourense, Spain; [email protected] 2 Clinical Pharmacology Group, Institute of Health Research (IDIS), Travesía da Choupana s/n, 15706 Santiago de Compostela, Spain; [email protected] (I.Z.-F.); [email protected] (M.G.-B.); [email protected] (E.B.-V.); [email protected] (V.M.S.) 3 Genomic Medicine Group, CIMUS, University of Santiago de Compostela, 15782 Santiago de Compostela, Spain; [email protected] (N.G.-A.); [email protected] (O.M.) 4 Pharmacy Department, University Clinical Hospital of Santiago de Compostela (SERGAS), Citation: Toja-Camba, F.J.; 15706 Santiago de Compostela, Spain; [email protected] Gesto-Antelo, N.; Maroñas, O.; 5 Department of Pharmacy and Pharmaceutical Technology and Parasitology, University of Valencia, Echarri Arrieta, E.; Zarra-Ferro, I.; 46100 Valencia, Spain González-Barcia, M.; Bandín-Vilar, E.; 6 Interuniversity Research Institute for Molecular Recognition and Technological Development, -
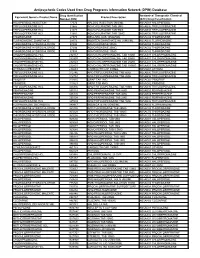
Antipsychotic Codes Used from Drug Programs Information Network (DPIN) Database
Antipsychotic Codes Used from Drug Programs Information Network (DPIN) Database Drug Identification Anatomical Therapeutic Chemical Equivalent Generic Product Name Product Description Number (DIN) (ATC) Drug Classification HALOPERIDOL INJECTION 17574 HALDOL INJECTION 5MG/ML N05AD01 HALOPERIDOL TRIFLUOPERAZINE HCL 21865 NOVO-FLURAZINE TAB 2MG N05AB06 TRIFLUOPERAZINE TRIFLUOPERAZINE HCL 21873 NOVO-FLURAZINE TAB 5MG N05AB06 TRIFLUOPERAZINE TRIFLUOPERAZINE HCL 21881 NOVO-FLURAZINE TAB 10MG N05AB06 TRIFLUOPERAZINE THIORIDAZINE 27375 MELLARIL SUS 10MG/5ML N05AC02 THIORIDAZINE FLUPHENAZINE ENANTHATE 29173 MODITEN ENANTHATE INJ 25MG/ML N05AB02 FLUPHENAZINE THIORIDAZINE HYDROCHLORIDE 37486 NOVO-RIDAZINE 50MG N05AC02 THIORIDAZINE THIORIDAZINE HYDROCHLORIDE 37494 NOVO-RIDAZINE 25MG N05AC02 THIORIDAZINE THIORIDAZINE HYDROCHLORIDE 37508 NOVO-RIDAZINE 10MG N05AC02 THIORIDAZINE CHLORPROMAZINE HCL 232157 NOVO-CHLORPROMAZINE TAB 10MG N05AA01 CHLORPROMAZINE CHLORPROMAZINE HCL 232807 NOVO-CHLORPROMAZINE TAB 50MG N05AA01 CHLORPROMAZINE CHLORPROMAZINE HCL 232823 NOVO-CHLORPROMAZINE TAB 25MG N05AA01 CHLORPROMAZINE CHLORPROMAZINE HCL 232831 NOVO-CHLORPROMAZINE TAB 100MG N05AA01 CHLORPROMAZINE LITHIUM CARBONATE 236683 CARBOLITH CAP 300MG N05AN01 LITHIUM TRIFLUOPERAZINE HCL 312746 APO TRIFLUOPERAZINE TAB 5MG N05AB06 TRIFLUOPERAZINE TRIFLUOPERAZINE HCL 312754 APO TRIFLUOPERAZINE TAB 2MG N05AB06 TRIFLUOPERAZINE PIMOZIDE 313815 ORAP TAB 2MG N05AG02 PIMOZIDE PIMOZIDE 313823 ORAP TAB 4MG N05AG02 PIMOZIDE TRIFLUOPERAZINE HCL 326836 APO TRIFLUOPERAZINE TAB 10MG N05AB06 -

Guidance on the Treatment of Antipsychotic Induced Hyperprolactinaemia in Adults
Guidance on the Treatment of Antipsychotic Induced Hyperprolactinaemia in Adults Version 1 GUIDELINE NO RATIFYING COMMITTEE DRUGS AND THERAPEUTICS GROUP DATE RATIFIED April 2014 DATE AVAILABLE ON INTRANET NEXT REVIEW DATE April 2016 POLICY AUTHORS Nana Tomova, Clinical Pharmacist Dr Richard Whale, Consultant Psychiatrist In association with: Dr Gordon Caldwell, Consultant Physician, WSHT . If you require this document in an alternative format, ie easy read, large text, audio, Braille or a community language, please contact the Pharmacy Team on 01243 623349 (Text Relay calls welcome). Contents Section Title Page Number 1. Introduction 2 2. Causes of Hyperprolactinaemia 2 3. Antipsychotics Associated with 3 Hyperprolactinaemia 4. Effects of Hyperprolactinaemia 4 5. Long-term Complications of Hyperprolactinaemia 4 5.1 Sexual Development in Adolescents 4 5.2 Osteoporosis 4 5.3 Breast Cancer 5 6. Monitoring & Baseline Prolactin Levels 5 7. Management of Hyperprolactinaemia 6 8. Pharmacological Treatment of 7 Hyperprolactinaemia 8.1 Aripiprazole 7 8.2 Dopamine Agonists 8 8.3 Oestrogen and Testosterone 9 8.4 Herbal Remedies 9 9. References 10 1 1.0 Introduction Prolactin is a hormone which is secreted from the lactotroph cells in the anterior pituitary gland under the influence of dopamine, which exerts an inhibitory effect on prolactin secretion1. A reduction in dopaminergic input to the lactotroph cells results in a rapid increase in prolactin secretion. Such a reduction in dopamine can occur through the administration of antipsychotics which act on dopamine receptors (specifically D2) in the tuberoinfundibular pathway of the brain2. The administration of antipsychotic medication is responsible for the high prevalence of hyperprolactinaemia in people with severe mental illness1.