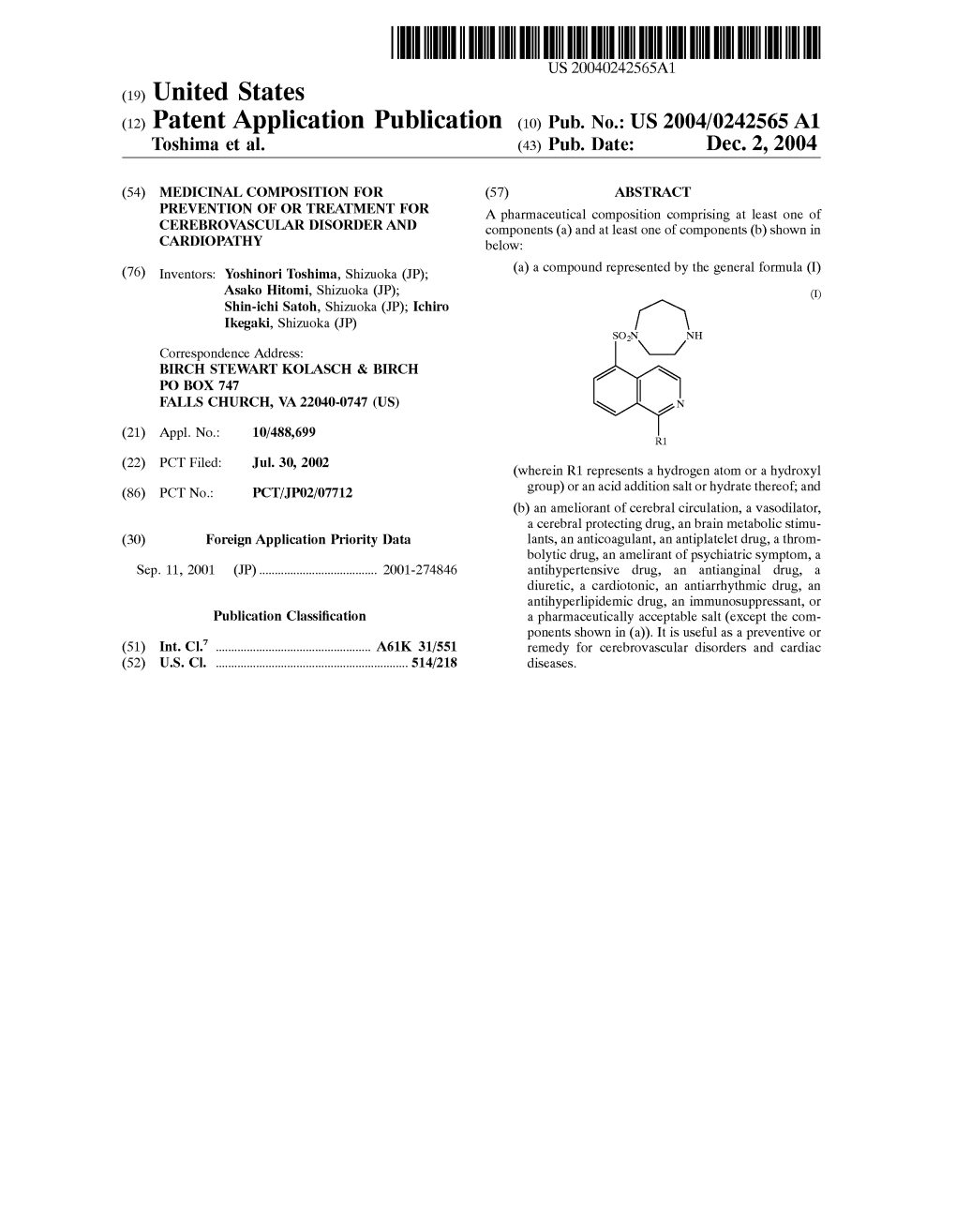
(12) Patent Application Publication (10) Pub. No.: US 2004/0242565 A1 Toshima Et Al
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

List of New Drugs Approved in India from 1991 to 2000
LIST OF NEW DRUGS APPROVED IN INDIA FROM 1991 TO 2000 S. No Name of Drug Pharmacological action/ Date of Indication Approval 1 Ciprofloxacin 0.3% w/v Eye Indicated in the treatment of February-1991 Drops/Eye Ointment/Ear Drop external ocular infection of the eye. 2 Diclofenac Sodium 1gm Gel March-1991 3 i)Cefaclor Monohydrate Antibiotic- In respiratory April-1991 250mg/500mg Capsule. infections, ENT infection, UT ii)Cefaclor Monohydrate infections, Skin and skin 125mg/5ml & 250mg/5ml structure infections. Suspension. iii)Cefaclor Monohydrate 100mg/ml Drops. iv)Cefaclor 187mg/5ml Suspension (For paediatric use). 4 Sheep Pox Vaccine (For April-1991 Veterinary) 5 Omeprazole 10mg/20mg Short term treatment of April-1991 Enteric Coated Granules duodenal ulcer, gastric ulcer, Capsule reflux oesophagitis, management of Zollinger- Ellison syndrome. 6 i)Nefopam Hydrochloride Non narcotic analgesic- Acute April-1991 30mg Tablet. and chronic pain, including ii)Nefopam Hydrochloride post-operative pain, dental 20mg/ml Injection. pain, musculo-skeletal pain, acute traumatic pain and cancer pain. 7 Buparvaquone 5% w/v Indicated in the treatment of April-1991 Solution for Injection (For bovine theileriosis. Veterinary) 8 i)Kitotifen Fumerate 1mg Anti asthmatic drug- Indicated May-1991 Tablet in prophylactic treatment of ii)Kitotifen Fumerate Syrup bronchial asthma, symptomatic iii)Ketotifen Fumerate Nasal improvement of allergic Drops conditions including rhinitis and conjunctivitis. 9 i)Pefloxacin Mesylate Antibacterial- In the treatment May-1991 Dihydrate 400mg Film Coated of severe infection in adults Tablet caused by sensitive ii)Pefloxacin Mesylate microorganism (gram -ve Dihydrate 400mg/5ml Injection pathogens and staphylococci). iii)Pefloxacin Mesylate Dihydrate 400mg I.V Bottles of 100ml/200ml 10 Ofloxacin 100mg/50ml & Indicated in RTI, UTI, May-1991 200mg/100ml vial Infusion gynaecological infection, skin/soft lesion infection. -

18512582.Pdf
European Heart Journal (2001) 22, 1938–1947 doi:10.1053/euhj.2001.2627, available online at http://www.idealibrary.com on The TRAPIST Study A multicentre randomized placebo controlled clinical trial of trapidil for prevention of restenosis after coronary stenting, measured by 3-D intravascular ultrasound P. W. Serruys1, D. P. Foley1, M. Pieper2, J. A. Kleijne3 and P. J. de Feyter1 on behalf of the TRAPIST investigators 1Department of Interventional Cardiology, Thoraxcenter, Erasmus Medical Center, Rotterdam, The Netherlands; 2Heartcenter Bodensee, Kreuzlingen, Switzerland; and 3Cardialysis, Rotterdam, The Netherlands Background Studies have reported benefit of oral therapy significant difference between trapidil and placebo-treated with the phosphodiesterase inhibitor, trapidil, in reducing patients regarding in-stent neointimal volume (108·6 restenosis after coronary angioplasty. Coronary stenting is 95·6 mm3 vs 93·379·1 mm3; P=0·16) or % obstruction associated with improved late outcome compared with volume (3818% vs 3621%; P=0·32), in angiographic balloon angioplasty, but significant neointimal hyperplasia minimal luminal diameter at follow-up (1·630·61 mm vs still occurs in a considerable proportion of patients. The 1·740·69 mm; P=0·17), restenosis rate (31% vs 24%; aim of this study was to investigate the safety and efficacy P=0·24), cumulative incidence of major adverse cardiac of trapidil 200 mg in preventing in-stent restenosis. events at 7 months (22% vs 20%; P=0·71) or anginal complaints (30% vs 24%; P=0·29). Methods Patients with a single native coronary lesion requiring revascularization were randomized to placebo or Conclusion Oral trapidil 600 mg daily for 6 months did trapidil at least 1 h before, and continuing for 6 months not reduce in-stent hyperplasia or improve clinical outcome after, successful implantation of a coronary Wallstent. -

Trapidil Inhibits Human Mesangiai Cell Proliferation: Effect on PDGF Β
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Elsevier - Publisher Connector Kidney International, Vol. 46 (1994), pp. 1002—1009 Trapidil inhibits human mesangial cell proliferation: Effect on PDGF a-receptor binding and expression LORETO GESUALDO, SALVATORE Di PAOLO, ELENA RANIERI, and FRANCESCO PAOLO SCHENA, with the technical assistance of ANNALISA BRUNACCINI Chair and Division of Nephrology, University of Bad, Ban, Italy Trapidil inhibits human mesangial cell proliferation: Effect on PDCF mediate, at least in part, the biological effect of other growth a-receptor binding and expression. Mesangial cell (MC) proliferation, a factors, as demonstrated for EGF, and elects PDGF as a potential histopathologic feature common to many human glomerular diseases, is regulated by several growth factors through their binding to specific cell autocrine regulator of MC proliferation [14]. surface receptors. Platelet-derived growth factor (PDGF) is a peptide MC play a pivotal role in the course of glomerular injury: they exerting a potent mitogenic activity on MC. Recently, an increasedfirst act as target cells for several mediators, which are released expression of both PDGF protein and its receptor has been localized in locally or are delivered through the systemic circulation [4, 15, 16]. the mesangial areas of several experimental as well as human proliferative Thereafter, they may drive the evolution of pathologic lesions glomerulonephritides (GN). Thus, it may be postulated that the inhibition of PDGF action could prevent MC proliferation during mesangial prolif- towards resolution or progression of the glomerular disease. MC erative GN. Trapidil, an antiplatelet drug, has been shown to inhibit the proliferation, a histopatologic feature common to different forms growth of several cell types both in vitro and in vivo. -

(12) United States Patent (10) Patent No.: US 7.803,838 B2 Davis Et Al
USOO7803838B2 (12) United States Patent (10) Patent No.: US 7.803,838 B2 Davis et al. (45) Date of Patent: Sep. 28, 2010 (54) COMPOSITIONS COMPRISING NEBIVOLOL 2002fO169134 A1 11/2002 Davis 2002/0177586 A1 11/2002 Egan et al. (75) Inventors: Eric Davis, Morgantown, WV (US); 2002/0183305 A1 12/2002 Davis et al. John O'Donnell, Morgantown, WV 2002/0183317 A1 12/2002 Wagle et al. (US); Peter Bottini, Morgantown, WV 2002/0183365 A1 12/2002 Wagle et al. (US) 2002/0192203 A1 12, 2002 Cho 2003, OOO4194 A1 1, 2003 Gall (73) Assignee: Forest Laboratories Holdings Limited 2003, OO13699 A1 1/2003 Davis et al. (BM) 2003/0027820 A1 2, 2003 Gall (*) Notice: Subject to any disclaimer, the term of this 2003.0053981 A1 3/2003 Davis et al. patent is extended or adjusted under 35 2003, OO60489 A1 3/2003 Buckingham U.S.C. 154(b) by 455 days. 2003, OO69221 A1 4/2003 Kosoglou et al. 2003/0078190 A1* 4/2003 Weinberg ...................... 514f1 (21) Appl. No.: 11/141,235 2003/0078517 A1 4/2003 Kensey 2003/01 19428 A1 6/2003 Davis et al. (22) Filed: May 31, 2005 2003/01 19757 A1 6/2003 Davis 2003/01 19796 A1 6/2003 Strony (65) Prior Publication Data 2003.01.19808 A1 6/2003 LeBeaut et al. US 2005/027281.0 A1 Dec. 8, 2005 2003.01.19809 A1 6/2003 Davis 2003,0162824 A1 8, 2003 Krul Related U.S. Application Data 2003/0175344 A1 9, 2003 Waldet al. (60) Provisional application No. 60/577,423, filed on Jun. -

Antithrombotic Treatment After Stroke Due to Intracerebral Haemorrhage (Review)
Cochrane Database of Systematic Reviews Antithrombotic treatment after stroke due to intracerebral haemorrhage (Review) Perry LA, Berge E, Bowditch J, Forfang E, Rønning OM, Hankey GJ, Villanueva E, Al-Shahi Salman R Perry LA, Berge E, Bowditch J, Forfang E, Rønning OM, Hankey GJ, Villanueva E, Al-Shahi Salman R. Antithrombotic treatment after stroke due to intracerebral haemorrhage. Cochrane Database of Systematic Reviews 2017, Issue 5. Art. No.: CD012144. DOI: 10.1002/14651858.CD012144.pub2. www.cochranelibrary.com Antithrombotic treatment after stroke due to intracerebral haemorrhage (Review) Copyright © 2017 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd. TABLE OF CONTENTS HEADER....................................... 1 ABSTRACT ...................................... 1 PLAINLANGUAGESUMMARY . 2 SUMMARY OF FINDINGS FOR THE MAIN COMPARISON . ..... 3 BACKGROUND .................................... 5 OBJECTIVES ..................................... 5 METHODS ...................................... 6 RESULTS....................................... 8 Figure1. ..................................... 9 Figure2. ..................................... 11 Figure3. ..................................... 12 DISCUSSION ..................................... 14 AUTHORS’CONCLUSIONS . 15 ACKNOWLEDGEMENTS . 15 REFERENCES ..................................... 15 CHARACTERISTICSOFSTUDIES . 18 DATAANDANALYSES. 31 Analysis 1.2. Comparison 1 Short-term antithrombotic treatment, Outcome 2 Death. 31 Analysis 1.6. Comparison 1 Short-term antithrombotic -

Partial Agreement in the Social and Public Health Field
COUNCIL OF EUROPE COMMITTEE OF MINISTERS (PARTIAL AGREEMENT IN THE SOCIAL AND PUBLIC HEALTH FIELD) RESOLUTION AP (88) 2 ON THE CLASSIFICATION OF MEDICINES WHICH ARE OBTAINABLE ONLY ON MEDICAL PRESCRIPTION (Adopted by the Committee of Ministers on 22 September 1988 at the 419th meeting of the Ministers' Deputies, and superseding Resolution AP (82) 2) AND APPENDIX I Alphabetical list of medicines adopted by the Public Health Committee (Partial Agreement) updated to 1 July 1988 APPENDIX II Pharmaco-therapeutic classification of medicines appearing in the alphabetical list in Appendix I updated to 1 July 1988 RESOLUTION AP (88) 2 ON THE CLASSIFICATION OF MEDICINES WHICH ARE OBTAINABLE ONLY ON MEDICAL PRESCRIPTION (superseding Resolution AP (82) 2) (Adopted by the Committee of Ministers on 22 September 1988 at the 419th meeting of the Ministers' Deputies) The Representatives on the Committee of Ministers of Belgium, France, the Federal Republic of Germany, Italy, Luxembourg, the Netherlands and the United Kingdom of Great Britain and Northern Ireland, these states being parties to the Partial Agreement in the social and public health field, and the Representatives of Austria, Denmark, Ireland, Spain and Switzerland, states which have participated in the public health activities carried out within the above-mentioned Partial Agreement since 1 October 1974, 2 April 1968, 23 September 1969, 21 April 1988 and 5 May 1964, respectively, Considering that the aim of the Council of Europe is to achieve greater unity between its members and that this -

The Use of Stems in the Selection of International Nonproprietary Names (INN) for Pharmaceutical Substances
WHO/PSM/QSM/2006.3 The use of stems in the selection of International Nonproprietary Names (INN) for pharmaceutical substances 2006 Programme on International Nonproprietary Names (INN) Quality Assurance and Safety: Medicines Medicines Policy and Standards The use of stems in the selection of International Nonproprietary Names (INN) for pharmaceutical substances FORMER DOCUMENT NUMBER: WHO/PHARM S/NOM 15 © World Health Organization 2006 All rights reserved. Publications of the World Health Organization can be obtained from WHO Press, World Health Organization, 20 Avenue Appia, 1211 Geneva 27, Switzerland (tel.: +41 22 791 3264; fax: +41 22 791 4857; e-mail: [email protected]). Requests for permission to reproduce or translate WHO publications – whether for sale or for noncommercial distribution – should be addressed to WHO Press, at the above address (fax: +41 22 791 4806; e-mail: [email protected]). The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the World Health Organization concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. Dotted lines on maps represent approximate border lines for which there may not yet be full agreement. The mention of specific companies or of certain manufacturers’ products does not imply that they are endorsed or recommended by the World Health Organization in preference to others of a similar nature that are not mentioned. Errors and omissions excepted, the names of proprietary products are distinguished by initial capital letters. -
![Ehealth DSI [Ehdsi V2.2.2-OR] Ehealth DSI – Master Value Set](https://docslib.b-cdn.net/cover/8870/ehealth-dsi-ehdsi-v2-2-2-or-ehealth-dsi-master-value-set-1028870.webp)
Ehealth DSI [Ehdsi V2.2.2-OR] Ehealth DSI – Master Value Set
MTC eHealth DSI [eHDSI v2.2.2-OR] eHealth DSI – Master Value Set Catalogue Responsible : eHDSI Solution Provider PublishDate : Wed Nov 08 16:16:10 CET 2017 © eHealth DSI eHDSI Solution Provider v2.2.2-OR Wed Nov 08 16:16:10 CET 2017 Page 1 of 490 MTC Table of Contents epSOSActiveIngredient 4 epSOSAdministrativeGender 148 epSOSAdverseEventType 149 epSOSAllergenNoDrugs 150 epSOSBloodGroup 155 epSOSBloodPressure 156 epSOSCodeNoMedication 157 epSOSCodeProb 158 epSOSConfidentiality 159 epSOSCountry 160 epSOSDisplayLabel 167 epSOSDocumentCode 170 epSOSDoseForm 171 epSOSHealthcareProfessionalRoles 184 epSOSIllnessesandDisorders 186 epSOSLanguage 448 epSOSMedicalDevices 458 epSOSNullFavor 461 epSOSPackage 462 © eHealth DSI eHDSI Solution Provider v2.2.2-OR Wed Nov 08 16:16:10 CET 2017 Page 2 of 490 MTC epSOSPersonalRelationship 464 epSOSPregnancyInformation 466 epSOSProcedures 467 epSOSReactionAllergy 470 epSOSResolutionOutcome 472 epSOSRoleClass 473 epSOSRouteofAdministration 474 epSOSSections 477 epSOSSeverity 478 epSOSSocialHistory 479 epSOSStatusCode 480 epSOSSubstitutionCode 481 epSOSTelecomAddress 482 epSOSTimingEvent 483 epSOSUnits 484 epSOSUnknownInformation 487 epSOSVaccine 488 © eHealth DSI eHDSI Solution Provider v2.2.2-OR Wed Nov 08 16:16:10 CET 2017 Page 3 of 490 MTC epSOSActiveIngredient epSOSActiveIngredient Value Set ID 1.3.6.1.4.1.12559.11.10.1.3.1.42.24 TRANSLATIONS Code System ID Code System Version Concept Code Description (FSN) 2.16.840.1.113883.6.73 2017-01 A ALIMENTARY TRACT AND METABOLISM 2.16.840.1.113883.6.73 2017-01 -

Search Strategy, Baseline Risk Coronavirus and Prophylaxis For
Supplement 7: Search Strategy, Baseline Risk Coronavirus and prophylaxis for thrombotic events Search narrative, 23 July 2020 Bibliographic databases: EMBASE (1974 to, 22 July 2020) Epistemonikos COVID-19 Evidence (21 July 2020) (includes records from Cochrane Database of Systematic Reviews, Pubmed, EMBASE, CINAHL, PsycINFO, LILACS, Database of reviews of Effects, The Campbell Collaboration online library, JBI Database of Systematic Reviews and Implementation Reports, EPPI-Centre Evidence Library) MEDLINE (1946 to, 23 July 2020) SCOPUS (19 July 2020) WHO Global Research Database (COVID-19) (20 July 2020) Trial/study Databases: Cochrane COVID-19 study register (23 July 2020) (includes records from ClinicalTrials.gov, WHO ICTRN, and PubMed) CYTEL map of ongoing [COVID-19] clinical trials (20 July 2020) (WHO International Clinical Trials Registry Platform, European Clinical Trials Registry, clinicaltrials.gov, Chinese Clinical Trial Registry, German Clinical Trials registry, Japan Primary Registries Network, Iranian Clinical Trial Registry, and Australian New Zealand Clinical Trials Registry) Results were downloaded from sources and deduplicated in EndNote. Results from BioRxiV, ChemRxiV, MedRxiV (preprints indexed in Medline and Epistemonikos), or protocols for clinical trials were removed as they were beyond the scope of the search. COCHRANE COVID-19 (https://covid-19.cochrane.org/) Search 1: anticoagula* or anti-coagula* or coagulation or thromb* or antithromb* or antiplatelet* or heparin or LMWH or aspirin or embol* or phlebothrombos* -

Pharmaceutical Appendix to the Tariff Schedule 2
Harmonized Tariff Schedule of the United States (2007) (Rev. 2) Annotated for Statistical Reporting Purposes PHARMACEUTICAL APPENDIX TO THE HARMONIZED TARIFF SCHEDULE Harmonized Tariff Schedule of the United States (2007) (Rev. 2) Annotated for Statistical Reporting Purposes PHARMACEUTICAL APPENDIX TO THE TARIFF SCHEDULE 2 Table 1. This table enumerates products described by International Non-proprietary Names (INN) which shall be entered free of duty under general note 13 to the tariff schedule. The Chemical Abstracts Service (CAS) registry numbers also set forth in this table are included to assist in the identification of the products concerned. For purposes of the tariff schedule, any references to a product enumerated in this table includes such product by whatever name known. ABACAVIR 136470-78-5 ACIDUM LIDADRONICUM 63132-38-7 ABAFUNGIN 129639-79-8 ACIDUM SALCAPROZICUM 183990-46-7 ABAMECTIN 65195-55-3 ACIDUM SALCLOBUZICUM 387825-03-8 ABANOQUIL 90402-40-7 ACIFRAN 72420-38-3 ABAPERIDONUM 183849-43-6 ACIPIMOX 51037-30-0 ABARELIX 183552-38-7 ACITAZANOLAST 114607-46-4 ABATACEPTUM 332348-12-6 ACITEMATE 101197-99-3 ABCIXIMAB 143653-53-6 ACITRETIN 55079-83-9 ABECARNIL 111841-85-1 ACIVICIN 42228-92-2 ABETIMUSUM 167362-48-3 ACLANTATE 39633-62-0 ABIRATERONE 154229-19-3 ACLARUBICIN 57576-44-0 ABITESARTAN 137882-98-5 ACLATONIUM NAPADISILATE 55077-30-0 ABLUKAST 96566-25-5 ACODAZOLE 79152-85-5 ABRINEURINUM 178535-93-8 ACOLBIFENUM 182167-02-8 ABUNIDAZOLE 91017-58-2 ACONIAZIDE 13410-86-1 ACADESINE 2627-69-2 ACOTIAMIDUM 185106-16-5 ACAMPROSATE 77337-76-9 -

Marrakesh Agreement Establishing the World Trade Organization
No. 31874 Multilateral Marrakesh Agreement establishing the World Trade Organ ization (with final act, annexes and protocol). Concluded at Marrakesh on 15 April 1994 Authentic texts: English, French and Spanish. Registered by the Director-General of the World Trade Organization, acting on behalf of the Parties, on 1 June 1995. Multilat ral Accord de Marrakech instituant l©Organisation mondiale du commerce (avec acte final, annexes et protocole). Conclu Marrakech le 15 avril 1994 Textes authentiques : anglais, français et espagnol. Enregistré par le Directeur général de l'Organisation mondiale du com merce, agissant au nom des Parties, le 1er juin 1995. Vol. 1867, 1-31874 4_________United Nations — Treaty Series • Nations Unies — Recueil des Traités 1995 Table of contents Table des matières Indice [Volume 1867] FINAL ACT EMBODYING THE RESULTS OF THE URUGUAY ROUND OF MULTILATERAL TRADE NEGOTIATIONS ACTE FINAL REPRENANT LES RESULTATS DES NEGOCIATIONS COMMERCIALES MULTILATERALES DU CYCLE D©URUGUAY ACTA FINAL EN QUE SE INCORPOR N LOS RESULTADOS DE LA RONDA URUGUAY DE NEGOCIACIONES COMERCIALES MULTILATERALES SIGNATURES - SIGNATURES - FIRMAS MINISTERIAL DECISIONS, DECLARATIONS AND UNDERSTANDING DECISIONS, DECLARATIONS ET MEMORANDUM D©ACCORD MINISTERIELS DECISIONES, DECLARACIONES Y ENTEND MIENTO MINISTERIALES MARRAKESH AGREEMENT ESTABLISHING THE WORLD TRADE ORGANIZATION ACCORD DE MARRAKECH INSTITUANT L©ORGANISATION MONDIALE DU COMMERCE ACUERDO DE MARRAKECH POR EL QUE SE ESTABLECE LA ORGANIZACI N MUND1AL DEL COMERCIO ANNEX 1 ANNEXE 1 ANEXO 1 ANNEX -

Total Thrombus-Formation Analysis System (T-TAS): Clinical Application of Quantitative Analysis of Thrombus Formation in Cardiovascular Disease
Published online: 2019-07-22 THIEME 1554 Theme Issue Article Total Thrombus-Formation Analysis System (T-TAS): Clinical Application of Quantitative Analysis of Thrombus Formation in Cardiovascular Disease Koichi Kaikita1 Kazuya Hosokawa2 Jeffrey R. Dahlen3 Kenichi Tsujita1 1 Department of Cardiovascular Medicine, Graduate School of Medical Address for correspondence Koichi Kaikita, MD, PhD, Department of Sciences, Kumamoto University, Kumamoto, Japan Cardiovascular Medicine, Graduate School of Medical Sciences, 2 Research Institute, Fujimori Kogyo Co., Yokohama, Kanagawa, Japan Kumamoto University, 1-1-1, Honjo, Chuo-ku, Kumamoto, 860-8556, 3 Hikari Dx, San Diego, California, United States Japan (e-mail: [email protected]). Thromb Haemost 2019;119:1554–1562. Abstract Various antithrombotic agents are clinically used to inhibit the cascade of arterial or venous thrombosis in cardiovascular diseases. Dual antiplatelet therapy with aspirin and P2Y12 inhibitors is prescribed in patients with coronary artery disease (CAD) undergoing percutaneous coronary intervention (PCI). Direct oral anticoagulants (DOACs) are widely used for the prevention or treatment of thromboembolism in patients with atrial fibrillation (AF) and venous thromboembolism. However, there has been no definitive tool to simultaneously monitor the antithrombotic effects of these drugs. The Total Thrombus-Formation Analysis System (T-TAS), a microchip-based flow chamber system that mimics in vivo conditions for evaluating whole blood thrombo- genicity, was developed for the quantitative analysis of thrombus formation in whole blood specimens. The utility of T-TAS has been evaluated in CAD patients treated with antiplatelet therapies. The T-TAS PL chip area under the flow pressure curve (AUC) accurately assesses primary hemostasis and is sensitive to the therapeutic effects of Keywords various antiplatelet therapies.