Evolution of Vascular Plants Through Redeployment of Ancient Developmental Regulators
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Gymnosperms the MESOZOIC: ERA of GYMNOSPERM DOMINANCE
Chapter 24 Gymnosperms THE MESOZOIC: ERA OF GYMNOSPERM DOMINANCE THE VASCULAR SYSTEM OF GYMNOSPERMS CYCADS GINKGO CONIFERS Pinaceae Include the Pines, Firs, and Spruces Cupressaceae Include the Junipers, Cypresses, and Redwoods Taxaceae Include the Yews, but Plum Yews Belong to Cephalotaxaceae Podocarpaceae and Araucariaceae Are Largely Southern Hemisphere Conifers THE LIFE CYCLE OF PINUS, A REPRESENTATIVE GYMNOSPERM Pollen and Ovules Are Produced in Different Kinds of Structures Pollination Replaces the Need for Free Water Fertilization Leads to Seed Formation GNETOPHYTES GYMNOSPERMS: SEEDS, POLLEN, AND WOOD THE ECOLOGICAL AND ECONOMIC IMPORTANCE OF GYMNOSPERMS The Origin of Seeds, Pollen, and Wood Seeds and Pollen Are Key Reproductive SUMMARY Innovations for Life on Land Seed Plants Have Distinctive Vegetative PLANTS, PEOPLE, AND THE Features ENVIRONMENT: The California Coast Relationships among Gymnosperms Redwood Forest 1 KEY CONCEPTS 1. The evolution of seeds, pollen, and wood freed plants from the need for water during reproduction, allowed for more effective dispersal of sperm, increased parental investment in the next generation and allowed for greater size and strength. 2. Seed plants originated in the Devonian period from a group called the progymnosperms, which possessed wood and heterospory, but reproduced by releasing spores. Currently, five lineages of seed plants survive--the flowering plants plus four groups of gymnosperms: cycads, Ginkgo, conifers, and gnetophytes. Conifers are the best known and most economically important group, including pines, firs, spruces, hemlocks, redwoods, cedars, cypress, yews, and several Southern Hemisphere genera. 3. The pine life cycle is heterosporous. Pollen strobili are small and seasonal. Each sporophyll has two microsporangia, in which microspores are formed and divide into immature male gametophytes while still retained in the microsporangia. -

"National List of Vascular Plant Species That Occur in Wetlands: 1996 National Summary."
Intro 1996 National List of Vascular Plant Species That Occur in Wetlands The Fish and Wildlife Service has prepared a National List of Vascular Plant Species That Occur in Wetlands: 1996 National Summary (1996 National List). The 1996 National List is a draft revision of the National List of Plant Species That Occur in Wetlands: 1988 National Summary (Reed 1988) (1988 National List). The 1996 National List is provided to encourage additional public review and comments on the draft regional wetland indicator assignments. The 1996 National List reflects a significant amount of new information that has become available since 1988 on the wetland affinity of vascular plants. This new information has resulted from the extensive use of the 1988 National List in the field by individuals involved in wetland and other resource inventories, wetland identification and delineation, and wetland research. Interim Regional Interagency Review Panel (Regional Panel) changes in indicator status as well as additions and deletions to the 1988 National List were documented in Regional supplements. The National List was originally developed as an appendix to the Classification of Wetlands and Deepwater Habitats of the United States (Cowardin et al.1979) to aid in the consistent application of this classification system for wetlands in the field.. The 1996 National List also was developed to aid in determining the presence of hydrophytic vegetation in the Clean Water Act Section 404 wetland regulatory program and in the implementation of the swampbuster provisions of the Food Security Act. While not required by law or regulation, the Fish and Wildlife Service is making the 1996 National List available for review and comment. -

1. TAKAKIACEAE S. Hattori & Inoue
1. TAKAKIACEAE S. Hattori & Inoue John R. Spence Wilfred B. Schofield Stems erect, arising sympodially from creeping pale or white stolons bearing clusters of beaked slime cells, rhizoids absent, stem in cross section differentiated into outer layer of smaller, thick- walled cells and cortex of larger thin-walled cells, sometimes with small central cells, these often with trigones, outer cells with simple oil droplets. Leaves typically 3-ranked, terete, forked, of (1–)2–4 terete segments, segments sometimes connate at base, in cross section of 3–5 cells, one or more larger central cells surrounded by smaller cells, oil droplets present in all cells, 2-celled slime hairs with enlarged apical cell present in axils of leaves. Specialized asexual reproduction by caducous leaves or stems. Sexual condition dioicous, gametangia naked. Seta with foot, persistent, elongating prior to capsule maturation. Capsule erect, symmetric, well-defined neck region absent, stomates absent, dextrorsely spiralled at maturity, columella ephemeral, basifixed, not penetrating the archesporial tissue, peristome absent, dehiscence by longitudinal helical slit. Calyptra fugacious to rarely persistent, typically mitrate. Spores 3-radiate, slightly roughened to papillose. Genus 1, species 2 (2 in the flora): North America, se Asia (including Borneo). Takakiaceae are plants of cool, cold-temperate to arctic-alpine oceanic climates. They were first collected in the Himalayas by J. D. Hooker and placed in the hepatic genus Lepidozia as L. ceratophylla by W. Mitten. The consensus has been that it was a highly unusual liverwort with affinity to the Calobryales. Distinctive features include erect shoots arising from a stolon, terete leaves, sometimes fused at or near the base and thus of 2–4 segments, naked lateral gametangia, slime cells, oil droplets, and chromosome numbers of n = 4 or 5. -

Early Evolution of the Land Plant Circadian Clock
Research Early evolution of the land plant circadian clock Anna-Malin Linde1,2*, D. Magnus Eklund1,2*, Akane Kubota3*, Eric R. A. Pederson1,2, Karl Holm1,2, Niclas Gyllenstrand1,2, Ryuichi Nishihama3, Nils Cronberg4, Tomoaki Muranaka5, Tokitaka Oyama5, Takayuki Kohchi3 and Ulf Lagercrantz1,2 1Department of Plant Ecology and Evolution, Evolutionary Biology Centre, Uppsala University, Norbyv€agen 18D, SE-75236 Uppsala, Sweden; 2The Linnean Centre for Plant Biology in Uppsala, Uppsala, Sweden; 3Graduate School of Biostudies, Kyoto University, Kyoto 606-8502, Japan; 4Department of Biology, Lund University, Ecology Building, SE-22362 Lund, Sweden; 5Graduate School of Science, Kyoto University, Kyoto 606-8502, Japan Summary Author for correspondence: While angiosperm clocks can be described as an intricate network of interlocked transcrip- Ulf Lagercrantz tional feedback loops, clocks of green algae have been modelled as a loop of only two genes. Tel: +46(0)18 471 6418 To investigate the transition from a simple clock in algae to a complex one in angiosperms, we Email: [email protected] performed an inventory of circadian clock genes in bryophytes and charophytes. Additionally, Received: 14 December 2016 we performed functional characterization of putative core clock genes in the liverwort Accepted: 18 January 2017 Marchantia polymorpha and the hornwort Anthoceros agrestis. Phylogenetic construction was combined with studies of spatiotemporal expression patterns New Phytologist (2017) 216: 576–590 and analysis of M. polymorpha clock gene mutants. doi: 10.1111/nph.14487 Homologues to core clock genes identified in Arabidopsis were found not only in bryophytes but also in charophytes, albeit in fewer copies. Circadian rhythms were detected Key words: bryophyte, circadian clock, for most identified genes in M. -

Patterns of Plant Diversity and Endemism in Namibia
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Stellenbosch University SUNScholar Repository Bothalia 36,2: 175-189(2006) Patterns of plant diversity and endemism in Namibia P. CRAVEN* and P VORSTER** Keywords: Namibia, phytogeography, plant endemism ABSTRACT Species richness, endemism and areas that are rich in both species and endemic species were assessed and mapped for Namibia. High species diversity corresponds with zones where species overlap. These are particularly obvious where there are altitudinal variations and in high-lying areas. The endemic flora o f Namibia is rich and diverse. An estimated 16% of the total plant species in Namibia are endemic to the country. Endemics are in a wide variety o f families and sixteen genera are endemic. Factors that increase the likelihood o f endemism are mountains, hot deserts, diversity o f substrates and microclimates. The distribution of plants endemic to Namibia was arranged in three different ways. Firstly, based on a grid count with the phytogeographic value of the species being equal, overall endemism was mapped. Secondly, range restricted plant species were mapped individually and those with congruent distribution patterns were combined. Thirdly, localities that are important for very range-restricted species were identified. The resulting maps of endemism and diversity were compared and found to correspond in many localities. When overall endemism is compared with overall diversity, rich localities may consist o f endemic species with wide ranges. The other methods identify important localities with their own distinctive complement of species. INTRODUCTION (1994). It was based on distributional data per magiste rial district following Merxmiiller (1966-1972), as well Species diversity was traditionally measured by count as other literature. -

Fern Gazette
ISSN 0308-0838 THE FERN GAZETTE VOLUME ELEVEN PART FIVE 1977 THE JOURNAL OF THE BRITISH PTERIDOLOGICAL SOCIETY THE FERN GAZETTE VOLUME 11 PART 5 1977 CONTENTS Page ECOLOGICAL NOTES Observations on some rare Spanish ferns iri Cadiz Province, Spain - B. Molesworth-AIIen 27 1 Unl:>ranched plants of Equisetum palustre L. - G. Halliday 276 Cyrtomium fa lcatum naturalised on Rhum - P. Corkh i/1 277 MAIN ARTICLES A pteridophyte flora of the Derbyshire Dales National Nature Reserve - A. Wil lmot 279 Ferns in the Cameroons. 11. The pteridophytes of the evergreen forests - G. Ben/ 285 An ecological survey of the ferns of the Canary Islands - C. N. Page 297 A new record of Synchytrium athyrii on Athyrium filix-femina - E. MUller & J.J. Schneller 313 Further cytogenetic studies and a reappraisal of the diploid ancestry in the Dryopteris carthusiana complex - M. Gibby & S. Wa lker 315 Cytology and reproduction of Ch eilanthes fa rinosa from Yemen -S.C. Verma 325 Lunathyrium in the Azores; a postscript- W.A. Sledge 33 1 SHORT NOTES Dryopteris x brathaica Fraser-Jenkins & Reichstein hybr.nov., the putative hybrid of D.carthusiana x D. fil ix-mas - C.R. Fraser-Jenkins & T.· Reichstein 337 No menclatural notes on Dryopteris - C.R. Fraser-Jenkins & A.C. Jermy 378 REVIEWS 278,329,341,342 [THE FERN GAZETTEVolum e 11 Part 4 was published 1st June 1976] Published by THE BRITISH PTERIDOLOGICAL SOCIETY, c/o Department of Botany, British Museum (Natural History), London SW7 5BD. FERN GAZ. 11(5) 1977 271 ECOLOGICAL NOTES OBSERVATIONS ON SOME RARE SPANISH FERNS IN CADIZ PROVINCE, SPAIN PTERIS SERRULATA Forskal. -
Plant Classification
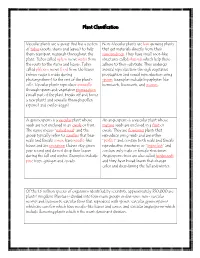
Plant Classification Vascular plants are a group that has a system Non-Vascular plants are low growing plants of tubes (roots, stems and leaves) to help that get materials directly from their them transport materials throughout the surroundings. They have small root-like plant. Tubes called xylem move water from structures called rhizoids which help them the roots to the stems and leaves. Tubes adhere to their substrate. They undergo called phloem move food from the leaves asexual reproduction through vegetative (where sugar is made during propagation and sexual reproduction using photosynthesis) to the rest of the plant’s spores. Examples include bryophytes like cells. Vascular plants reproduce asexually hornworts, liverworts, and mosses. through spores and vegetative propagation (small part of the plant breaks off and forms a new plant) and sexually through pollen (sperm) and ovules (eggs). A gymnosperm is a vascular plant whose An angiosperm is a vascular plant whose seeds are not enclosed in an ovule or fruit. mature seeds are enclosed in a fruit or The name means “naked seed” and the ovule. They are flowering plants that group typically refers to conifers that bear reproduce using seeds and are either male and female cones, have needle-like “perfect” and contain both male and female leaves and are evergreen (leaves stay green reproductive structures or “imperfect” and year round and do not drop their leaves contain only male or female structures. during the fall and winter. Examples include Angiosperm trees are also called hardwoods pine trees, ginkgos and cycads. and they have broad leaves that change color and drop during the fall and winter. -

JUDD W.S. Et. Al. (2002) Plant Systematics: a Phylogenetic Approach. Chapter 7. an Overview of Green
UNCORRECTED PAGE PROOFS An Overview of Green Plant Phylogeny he word plant is commonly used to refer to any auto- trophic eukaryotic organism capable of converting light energy into chemical energy via the process of photosynthe- sis. More specifically, these organisms produce carbohydrates from carbon dioxide and water in the presence of chlorophyll inside of organelles called chloroplasts. Sometimes the term plant is extended to include autotrophic prokaryotic forms, especially the (eu)bacterial lineage known as the cyanobacteria (or blue- green algae). Many traditional botany textbooks even include the fungi, which differ dramatically in being heterotrophic eukaryotic organisms that enzymatically break down living or dead organic material and then absorb the simpler products. Fungi appear to be more closely related to animals, another lineage of heterotrophs characterized by eating other organisms and digesting them inter- nally. In this chapter we first briefly discuss the origin and evolution of several separately evolved plant lineages, both to acquaint you with these important branches of the tree of life and to help put the green plant lineage in broad phylogenetic perspective. We then focus attention on the evolution of green plants, emphasizing sev- eral critical transitions. Specifically, we concentrate on the origins of land plants (embryophytes), of vascular plants (tracheophytes), of 1 UNCORRECTED PAGE PROOFS 2 CHAPTER SEVEN seed plants (spermatophytes), and of flowering plants dons.” In some cases it is possible to abandon such (angiosperms). names entirely, but in others it is tempting to retain Although knowledge of fossil plants is critical to a them, either as common names for certain forms of orga- deep understanding of each of these shifts and some key nization (e.g., the “bryophytic” life cycle), or to refer to a fossils are mentioned, much of our discussion focuses on clade (e.g., applying “gymnosperms” to a hypothesized extant groups. -

A Journal on Taxonomic Botany, Plant Sociology and Ecology Reinwardtia
A JOURNAL ON TAXONOMIC BOTANY, PLANT SOCIOLOGY AND ECOLOGY REINWARDTIA A JOURNAL ON TAXONOMIC BOTANY, PLANT SOCIOLOGY AND ECOLOGY Vol. 13(4): 317 —389, December 20, 2012 Chief Editor Kartini Kramadibrata (Herbarium Bogoriense, Indonesia) Editors Dedy Darnaedi (Herbarium Bogoriense, Indonesia) Tukirin Partomihardjo (Herbarium Bogoriense, Indonesia) Joeni Setijo Rahajoe (Herbarium Bogoriense, Indonesia) Teguh Triono (Herbarium Bogoriense, Indonesia) Marlina Ardiyani (Herbarium Bogoriense, Indonesia) Eizi Suzuki (Kagoshima University, Japan) Jun Wen (Smithsonian Natural History Museum, USA) Managing editor Himmah Rustiami (Herbarium Bogoriense, Indonesia) Secretary Endang Tri Utami Lay out editor Deden Sumirat Hidayat Illustrators Subari Wahyudi Santoso Anne Kusumawaty Reviewers Ed de Vogel (Netherlands), Henk van der Werff (USA), Irawati (Indonesia), Jan F. Veldkamp (Netherlands), Jens G. Rohwer (Denmark), Lauren M. Gardiner (UK), Masahiro Kato (Japan), Marshall D. Sunberg (USA), Martin Callmander (USA), Rugayah (Indonesia), Paul Forster (Australia), Peter Hovenkamp (Netherlands), Ulrich Meve (Germany). Correspondence on editorial matters and subscriptions for Reinwardtia should be addressed to: HERBARIUM BOGORIENSE, BOTANY DIVISION, RESEARCH CENTER FOR BIOLOGY-LIPI, CIBINONG 16911, INDONESIA E-mail: [email protected] REINWARDTIA Vol 13, No 4, pp: 367 - 377 THE NEW PTERIDOPHYTE CLASSIFICATION AND SEQUENCE EM- PLOYED IN THE HERBARIUM BOGORIENSE (BO) FOR MALESIAN FERNS Received July 19, 2012; accepted September 11, 2012 WITA WARDANI, ARIEF HIDAYAT, DEDY DARNAEDI Herbarium Bogoriense, Botany Division, Research Center for Biology-LIPI, Cibinong Science Center, Jl. Raya Jakarta -Bogor Km. 46, Cibinong 16911, Indonesia. E-mail: [email protected] ABSTRACT. WARD AM, W., HIDAYAT, A. & DARNAEDI D. 2012. The new pteridophyte classification and sequence employed in the Herbarium Bogoriense (BO) for Malesian ferns. -

Extant Diversity of Bryophytes Emerged from Successive Post-Mesozoic Diversification Bursts
ARTICLE Received 20 Mar 2014 | Accepted 3 Sep 2014 | Published 27 Oct 2014 DOI: 10.1038/ncomms6134 Extant diversity of bryophytes emerged from successive post-Mesozoic diversification bursts B. Laenen1,2, B. Shaw3, H. Schneider4, B. Goffinet5, E. Paradis6,A.De´samore´1,2, J. Heinrichs7, J.C. Villarreal7, S.R. Gradstein8, S.F. McDaniel9, D.G. Long10, L.L. Forrest10, M.L. Hollingsworth10, B. Crandall-Stotler11, E.C. Davis9, J. Engel12, M. Von Konrat12, E.D. Cooper13, J. Patin˜o1, C.J. Cox14, A. Vanderpoorten1,* & A.J. Shaw3,* Unraveling the macroevolutionary history of bryophytes, which arose soon after the origin of land plants but exhibit substantially lower species richness than the more recently derived angiosperms, has been challenged by the scarce fossil record. Here we demonstrate that overall estimates of net species diversification are approximately half those reported in ferns and B30% those described for angiosperms. Nevertheless, statistical rate analyses on time- calibrated large-scale phylogenies reveal that mosses and liverworts underwent bursts of diversification since the mid-Mesozoic. The diversification rates further increase in specific lineages towards the Cenozoic to reach, in the most recently derived lineages, values that are comparable to those reported in angiosperms. This suggests that low diversification rates do not fully account for current patterns of bryophyte species richness, and we hypothesize that, as in gymnosperms, the low extant bryophyte species richness also results from massive extinctions. 1 Department of Conservation Biology and Evolution, Institute of Botany, University of Lie`ge, Lie`ge 4000, Belgium. 2 Institut fu¨r Systematische Botanik, University of Zu¨rich, Zu¨rich 8008, Switzerland. -

Volume 1, Chapter 2-4: Bryophyta
Glime, J. M. 2017. Bryophyta - Takakiopsida. Chapt. 2-4. In: Glime, J. M. Bryophyte Ecology. Volume 1. Physiological Ecology. 2-4-1 Ebook sponsored by Michigan Technological University and the International Association of Bryologists. Last updated 9 April 2021 and available at <http://digitalcommons.mtu.edu/bryophyte-ecology/>. CHAPTER 2-4 BRYOPHYTA – TAKAKIOPSIDA TABLE OF CONTENTS Phylum Bryophyta .............................................................................................................................................. 2-4-2 Class Takakiopsida...................................................................................................................................... 2-4-2 Summary ........................................................................................................................................................... 2-4-10 Acknowledgments............................................................................................................................................. 2-4-10 Literature Cited ................................................................................................................................................. 2-4-10 2-4-2 Chapter 2-4: Bryophyta - Takakiopsida CHAPTER 2-4 BRYOPHYTA – TAKAKIOPSIDA Figure 1. Mt. Daisetsu from Kogan Spa, Hokkaido, Japan. The foggy peak of Mt. Daisetsu is the home of Takakia lepidozioides. Photo by Janice Glime. the Bryopsida, Andreaeopsida, and Sphagnopsida (Crum 1991). However, as more evidence from genetic and biochemical relationships -

Fern Classification
16 Fern classification ALAN R. SMITH, KATHLEEN M. PRYER, ERIC SCHUETTPELZ, PETRA KORALL, HARALD SCHNEIDER, AND PAUL G. WOLF 16.1 Introduction and historical summary / Over the past 70 years, many fern classifications, nearly all based on morphology, most explicitly or implicitly phylogenetic, have been proposed. The most complete and commonly used classifications, some intended primar• ily as herbarium (filing) schemes, are summarized in Table 16.1, and include: Christensen (1938), Copeland (1947), Holttum (1947, 1949), Nayar (1970), Bierhorst (1971), Crabbe et al. (1975), Pichi Sermolli (1977), Ching (1978), Tryon and Tryon (1982), Kramer (in Kubitzki, 1990), Hennipman (1996), and Stevenson and Loconte (1996). Other classifications or trees implying relationships, some with a regional focus, include Bower (1926), Ching (1940), Dickason (1946), Wagner (1969), Tagawa and Iwatsuki (1972), Holttum (1973), and Mickel (1974). Tryon (1952) and Pichi Sermolli (1973) reviewed and reproduced many of these and still earlier classifica• tions, and Pichi Sermolli (1970, 1981, 1982, 1986) also summarized information on family names of ferns. Smith (1996) provided a summary and discussion of recent classifications. With the advent of cladistic methods and molecular sequencing techniques, there has been an increased interest in classifications reflecting evolutionary relationships. Phylogenetic studies robustly support a basal dichotomy within vascular plants, separating the lycophytes (less than 1 % of extant vascular plants) from the euphyllophytes (Figure 16.l; Raubeson and Jansen, 1992, Kenrick and Crane, 1997; Pryer et al., 2001a, 2004a, 2004b; Qiu et al., 2006). Living euphyl• lophytes, in turn, comprise two major clades: spermatophytes (seed plants), which are in excess of 260 000 species (Thorne, 2002; Scotland and Wortley, Biology and Evolution of Ferns and Lycopliytes, ed.