The Role of Molecular Motors in Peripheral Nerve Regeneration
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
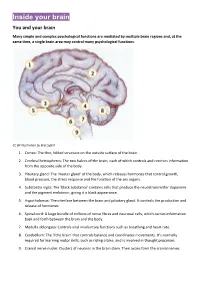
Inside Your Brain You and Your Brain
Inside your brain You and your brain Many simple and complex psychological functions are mediated by multiple brain regions and, at the same time, a single brain area may control many psychological functions. CC BY Illustration by Bret Syfert 1. Cortex: The thin, folded structure on the outside surface of the brain. 2. Cerebral hemispheres: The two halves of the brain, each of which controls and receives information from the opposite side of the body. 3. Pituitary gland: The ‘master gland’ of the body, which releases hormones that control growth, blood pressure, the stress response and the function of the sex organs. 4. Substantia nigra: The ‘black substance’ contains cells that produce the neurotransmitter dopamine and the pigment melatonin, giving it a black appearance. 5. Hypothalamus: The interface between the brain and pituitary gland. It controls the production and release of hormones. 6. Spinal cord: A large bundle of millions of nerve fibres and neuronal cells, which carries information back and forth between the brain and the body. 7. Medulla oblongata: Controls vital involuntary functions such as breathing and heart rate. 8. Cerebellum: The ‘little brain’ that controls balance and coordinates movements. It’s normally required for learning motor skills, such as riding a bike, and is involved in thought processes. 9. Cranial nerve nuclei: Clusters of neurons in the brain stem. Their axons form the cranial nerves. Your brain underpins who you are. It stores your knowledge and memories, gives you the capacity for thought and emotion, and enables you to control your body. The brain is just one part of the nervous system. -

Retrograde Transport of Plasticity Signals in Ap/Ysia Sensory Neurons Following Axonal Injury
The Journal of Neuroscience, January 1995, 15(i): 439-448 Retrograde Transport of Plasticity Signals in Ap/ysia Sensory Neurons Following Axonal Injury John D. Gunstream, Gilbert A. Castro, and Edgar T. Walters Department of Physiology and Cell Biology, University of Texas-Houston Medical School, Houston, Texas 77225 Following injury to their peripheral branches, mechanosen- 1990). Becausemany of these alterations involve changesin sory neurons in Aplysia display long-term plasticity that is geneexpression and protein synthesis(e.g., Watson, 1968; Her- expressed as soma hyperexcitability, synaptic facilitation, degen et al., 1992; Haas et al., 1993) pathways must exist that and neurite outgrowth. To investigate the nature of signals inform the nucleus of cellular damage occurring far from the that convey information about distant axonal injury, we have soma. Many potential injury signalsexist, which may act singly investigated the development of injury-induced soma hy- or in concert (e.g., Cragg, 1970; Lieberman, 1971; Aldskogius perexcitability in two in vitro preparations. In isolated gan- et al., 1992). However, it is useful to distinguish two classesof glia, proximal nerve crush caused hyperexcitability to ap- axonal signals (e.g., Wu et al., 1993). Negative injury signals pear sooner than did distal crush, and the difference in result from an interruption of retrograde transport of chemical development of hyperexcitability indicated that the injury signals,such as trophic substances,that normally are continu- signal moved at a rate (36 mm/d) similar to previously re- ously conveyed from distal parts of an uninjured axon to the ported rates of retrograde axonal transport in this animal. -

Chapter 4 : Neuronopathies and Axonopathies
CHAPTER 4 : NEURONOPATHIES AND AXONOPATHIES Chapter 4.1 CLASSIFICATION This group of conditions is characterised by selective non-inflammatory, neuronal degeneration involving either neurons in their entirety (neuronopathies) or axons in a more restricted manner (axonopathies). Division between these two categories may be difficult purely on morphological grounds as the axon is a dependant part of the neuron [1]. Jubb & Huxtable subclassify these conditions into three categories according to the distribution of the lesions in the central and peripheral nervous system: • central neuronopathies and axonopathies (e.g. organomercurial poisoning, congenital axonopathy in Holstein-Friesian calves and the axonal dystrophies), • central and peripheral neuronopathies and axonopathies (e.g. organophosphate poisoning, neonatal copper deficiency and neurodegeneration of Horned Hereford calves), and • peripheral axonopathies (uncommon and mostly reported in the horse e.g. equine laryngeal hemiplegia and equine stringhalt) Reference 1. Jubb KVF, Huxtable CR (1993) The Nervous System. In: Jubb KVF, Kennedy PC, Palmer N (eds) Pathology of Domestic Animals 4th edn Academic Press, Inc, San Diego, pp 267- 439 129 Chapter 4.2 ACUTE ASPERGILLUS CLAVATUS POISONING IN CATTLE: LIGHT MICROSCOPICAL AND ULTRASTRUCTURAL LESIONS IN THE SPINAL CORD JJ van der Lugt1, L Prozesky2, E van Wilpe3 1Department of Paraclinical Sciences, Faculty of Veterinary Science, University of Pretoria, Private Bag X05, 0110 Onderstepoort, South Africa. Present address: Department of -

Microglia and Macrophages in the Pathological Central and Peripheral Nervous Systems
cells Review Microglia and Macrophages in the Pathological Central and Peripheral Nervous Systems Naoki Abe 1, Tasuku Nishihara 1,*, Toshihiro Yorozuya 1 and Junya Tanaka 2 1 Department of Anesthesia and Perioperative Medicine, Ehime University Graduate School of Medicine, Toon, Ehime 791-0295, Japan; [email protected] (N.A.); [email protected] (T.Y.) 2 Department of Molecular and cellular Physiology, Ehime University Graduate School of Medicine, Toon, Ehime 791-0295, Japan; [email protected] * Correspondence: [email protected]; Tel.: +81-89-960-5383; Fax: +81-89-960-5386 Received: 4 August 2020; Accepted: 17 September 2020; Published: 21 September 2020 Abstract: Microglia, the immunocompetent cells in the central nervous system (CNS), have long been studied as pathologically deteriorating players in various CNS diseases. However, microglia exert ameliorating neuroprotective effects, which prompted us to reconsider their roles in CNS and peripheral nervous system (PNS) pathophysiology. Moreover, recent findings showed that microglia play critical roles even in the healthy CNS. The microglial functions that normally contribute to the maintenance of homeostasis in the CNS are modified by other cells, such as astrocytes and infiltrated myeloid cells; thus, the microglial actions on neurons are extremely complex. For a deeper understanding of the pathophysiology of various diseases, including those of the PNS, it is important to understand microglial functioning. In this review, we discuss both the favorable and unfavorable roles of microglia in neuronal survival in various CNS and PNS disorders. We also discuss the roles of blood-borne macrophages in the pathogenesis of CNS and PNS injuries because they cooperatively modify the pathological processes of resident microglia. -

Gamma Motor Neurons Survive and Exacerbate Alpha Motor Neuron Degeneration In
Gamma motor neurons survive and exacerbate alpha PNAS PLUS motor neuron degeneration in ALS Melanie Lalancette-Heberta,b, Aarti Sharmaa,b, Alexander K. Lyashchenkoa,b, and Neil A. Shneidera,b,1 aCenter for Motor Neuron Biology and Disease, Columbia University, New York, NY 10032; and bDepartment of Neurology, Columbia University, New York, NY 10032 Edited by Rob Brownstone, University College London, London, United Kingdom, and accepted by Editorial Board Member Fred H. Gage October 27, 2016 (received for review April 4, 2016) The molecular and cellular basis of selective motor neuron (MN) the muscle spindle and control the sensitivity of spindle afferent vulnerability in amyotrophic lateral sclerosis (ALS) is not known. In discharge (15); beta (β) skeletofusimotor neurons innervate both genetically distinct mouse models of familial ALS expressing intra- and extrafusal muscle (16). In addition to morphological mutant superoxide dismutase-1 (SOD1), TAR DNA-binding protein differences, distinct muscle targets, and the absence of primary 43 (TDP-43), and fused in sarcoma (FUS), we demonstrate selective afferent (IA)inputsonγ-MNs, these functional MN subtypes also degeneration of alpha MNs (α-MNs) and complete sparing of differ in their trophic requirements, and γ-MNs express high levels gamma MNs (γ-MNs), which selectively innervate muscle spindles. of the glial cell line-derived neurotropic factor (GDNF) receptor Resistant γ-MNs are distinct from vulnerable α-MNs in that they Gfrα1 (17). γ-MNs are also molecularly distinguished by the ex- lack synaptic contacts from primary afferent (IA) fibers. Elimination pression of other selective markers including the transcription α of these synapses protects -MNs in the SOD1 mutant, implicating factor Err3 (18), Wnt7A (19), the serotonin receptor 1d (5-ht1d) this excitatory input in MN degeneration. -

Microglia and C9orf72 in Neuroinflammation and ALS and Frontotemporal Dementia
Microglia and C9orf72 in neuroinflammation and ALS and frontotemporal dementia Deepti Lall, Robert H. Baloh J Clin Invest. 2017;127(9):3250-3258. https://doi.org/10.1172/JCI90607. Review Series Amyotrophic lateral sclerosis (ALS) is a degenerative disorder that is characterized by loss of motor neurons and shows clinical, pathological, and genetic overlap with frontotemporal dementia (FTD). Activated microglia are a universal feature of ALS/FTD pathology; however, their role in disease pathogenesis remains incompletely understood. The recent discovery that ORF 72 on chromosome 9 (C9orf72), the gene most commonly mutated in ALS/FTD, has an important role in myeloid cells opened the possibility that altered microglial function plays an active role in disease. This Review highlights the contribution of microglia to ALS/FTD pathogenesis, discusses the connection between autoimmunity and ALS/FTD, and explores the possibility that C9orf72 and other ALS/FTD genes may have a “dual effect” on both neuronal and myeloid cell function that could explain a shared propensity for altered systemic immunity and neurodegeneration. Find the latest version: https://jci.me/90607/pdf REVIEW SERIES: GLIA AND NEURODEGENERATION The Journal of Clinical Investigation Series Editors: Marco Colonna and David Holtzmann Microglia and C9orf72 in neuroinflammation and ALS and frontotemporal dementia Deepti Lall1 and Robert H. Baloh1,2 1Board of Governors Regenerative Medicine Institute and 2Department of Neurology, Cedars-Sinai Medical Center, Los Angeles, California, USA. Amyotrophic lateral sclerosis (ALS) is a degenerative disorder that is characterized by loss of motor neurons and shows clinical, pathological, and genetic overlap with frontotemporal dementia (FTD). Activated microglia are a universal feature of ALS/FTD pathology; however, their role in disease pathogenesis remains incompletely understood. -

ARIA Is Concentrated in Nerve Terminals at Neuromuscular Junctions and at Other Synapses
The Journal of Neuroscience, September 1995, 15(g): 6124-6136 ARIA Is Concentrated in Nerve Terminals at Neuromuscular Junctions and at Other Synapses Alfred W. Sandrock, Jr.,i,2 Andrew D. J. Goodearl,’ Qin-Wei Yin,’ David Chang,3 and Gerald D. Fischbachl ‘Department of Neurobiology, Harvard Medical School, Boston, Massachusetts 02115, “Department of Neurology, Massachusetts General Hosoital, Boston, Massachusetts 02114, and 3Amgen, Inc., Thousand Oaks, California 91320 Skeletal muscle ACh receptors (AChRs) accumulate at neu- natal maturation of AChRs at the neuromuscular junction, which romuscular junctions (nmjs) at least partly because of the results in the replacement of y-subunit-containing receptors with selective induction of AChR subunit genes in subsynaptic those containing E- subunits (Brenner and Sakmann, 1978; Bren- myotube nuclei by the motor nerve terminal. Additionally, ner et al., 1990; Martinou and Merlie, 1991). ARIA (for ACh mammalian AChRs undergo a postnatal change in subunit receptor inducing activity), which was purified from chicken composition from embryonic (cw.&S) to adult (@eS) brains based on its ability to stimulate the synthesis of AChRs forms, a switch that also depends on innervation. ARIA, a in skeletal muscle (Falls et al., 1993), may mediate the ability protein purified from chicken brains based on its ability to of the motor neuron to orchestrate these developmental events. induce AChR synthesis in primary chick muscle cells, is a ARIA mRNA is concentrated in chick and rat motor neurons strong candidate for being the molecule responsible for (Falls et al., 1993; Corfas et al., 1995), and, in both species, is these early developmental events. -

Perineurial Glia Require Notch Signaling During Motor Nerve Development but Not Regeneration
The Journal of Neuroscience, March 6, 2013 • 33(10):4241–4252 • 4241 Development/Plasticity/Repair Perineurial Glia Require Notch Signaling during Motor Nerve Development but Not Regeneration Laura A. Binari, Gwendolyn M. Lewis, and Sarah Kucenas Department of Biology, University of Virginia, Charlottesville, Virginia 22904 Motor nerves play the critical role of shunting information out of the CNS to targets in the periphery. Their formation requires the coordinated development of distinct cellular components, including motor axons and the Schwann cells and perineurial glia that en- sheath them. During nervous system assembly, these glial cells must migrate long distances and terminally differentiate, ensuring the efficient propagation of action potentials. Although we know quite a bit about the mechanisms that control Schwann cell development during this process, nothing is known about the mechanisms that mediate the migration and differentiation of perineurial glia. Using in vivo imaging in zebrafish, we demonstrate that Notch signaling is required for both perineurial migration and differentiation during nerve formation, but not regeneration. Interestingly, loss of Notch signaling in perineurial cells also causes a failure of Schwann cell differentiation, demonstrating that Schwann cells require perineurial glia for aspects of their own development. These studies describe a novel mechanism that mediates multiple aspects of perineurial development and reveal the critical importance of perineurial glia for Schwann cell maturation and nerve formation. Introduction independent. However, to date, nothing is known about these A fundamental goal in neuroscience is to understand what con- nonaxonal mechanisms. trols cell migration. Unlike other organ systems where cells are In zebrafish, perineurial glia originate from precursors in restricted to a discrete space, peripheral glia must migrate long the floor plate (p3 domain) of the spinal cord, migrate out of distances from their origin to their target. -

Axonal and Perikaryal Involvement in Chronic Inflammatory Demyelinating
J Neurol Neurosurg Psychiatry 1999;66:727–734 727 J Neurol Neurosurg Psychiatry: first published as 10.1136/jnnp.66.6.727 on 1 June 1999. Downloaded from Axonal and perikaryal involvement in chronic inflammatory demyelinating polyneuropathy M Nagamatsu, S Terao, K Misu, M Li, N Hattori, M Ichimura, M Sakai, H Yamamoto, H Watanabe, S Riku, E Ikeda, J Hata, M Oda, M Satake, N Nakamura, S Matsuya, Y Hashizume, G Sobue Abstract ery. In this study, we assessed the degree of Department of Objectives—To assess the extent of loss of involvement of spinal motor neurons and Neurology, Nagoya peripheral nerve axons in CIDP. University School of myelinated nerve fibres and spinal motor Medicine, Nagoya, neuron loss in chronic inflammatory de- Japan myelinating polyneuropathy (CIDP), a M Nagamatsu clinicopathological study was conducted Methods MLi on biopsied sural nerves and necropsied SPECIMENS K Misu spinal cords from patients with CIDP. After informed consent was given, sural nerve N Hattori biopsy specimens from 71 patients with CIDP Methods—The myelinated fibre pathology M Ichimura (50 males and 21 females) were obtained at the G Sobue of 71 biopsied sural nerves and motor neuron pathology of nine necropsied spi- Nagoya University School of Medicine and its a liated hospitals over 11 years. Age at biopsy Fourth Department of nal cords at L4 levels in patients with Y ranged from 2 to 81 years; mean (SD) age 48.5 Internal Medicine, CIDP were quantitatively and immuno- Aichi Medical (21.9) years. The duration of illness before histochemically assessed. University, Aichi, biopsy ranged from 2 months to 28 years; mean —Myelinated nerve fibre density Japan Results (SD) 2.9 (5.8) years. -

Phosphorylation of Neurofilament Proteins and Chromatolysis Following Transection of Rat Sciatic Nerve
The Journal of Neuroscience, May 1987, 7(5): 1588-1594 Phosphorylation of Neurofilament Proteins and Chromatolysis Following Transection of Rat Sciatic Nerve Margi E. Goldstein, Harold S. Cooper, Jennifer Bruce, Martin J. Carden, Virginia M.-Y. Lee, and William W. Schlaepfer Division of Neuropathology, Department of Pathology and Laboratory Medicine, University of Pennsylvania Medical School, Philadelphia, Pennsylvania 19104 States of phosphorylation of neurofilament proteins were peripheral margination of Nissl substance, nuclear displace- examined in the perikarya of rat sensory and motor neurons ment, and nucleolar enlargement(Guth, 1956; Kirkpatrick, 1968; between 3 and 28 d following either a distal transection [6- Lieberman, 1971; Grafstein, 1975; Torvik, 1976; Hughs, 1978; 7 cm from the L4-L5 dorsal root ganglia (DRG)] or a proximal Tennyson and Gershon, 1984) are manifestations of the cell’s transection (l-2 cm from the L4-L5 DRG) of the sciatic nerve. attempt to regeneratethe injured axon (Cragg, 1970; Price and Paraffin sections of the right (experimental) and left (control) Porter, 1972; Grafstein, 1975; Torvik, 1976; Hall et al., 1978). L4 and L5 DRG from animals with unilateral transection of The dependenceof these changeson the type and location of the right distal sciatic nerve were stained immunocytochem- the lesion (Humbertson, 1963; Watson, 1968; Lieberman, 1971; ically with monoclonal antibodies to phosphorylation-de- Torvik and Skjorten, 197 1; Torvik, 1976; Aldskogius and Ar- pendent (NF-P), dephosphorylation-dependent (NF-dP), or vidsson, 1978; Hall et al., 1978; Hughs, 1978; Sterman and phosphorylation-independent (NF-ind) epitopes on the larg- Delannoy, 1985) indicates that the chromatolytic reaction is est (NF200), mid-sized (NF150), or smallest (NF68) neuro- mediated by retrograde signals from the site of injury (Cava- filament protein subunits. -

Vacuolation of Chromatolysis of Lower Motoneurons in Tetanus
Case report Vacuolation and chromatolysis of lower motoneurons in tetanus A case report and review of the literature In recent years, the pathophysiological action of tetanus toxin has been extensively studied by many investigators.1,2 The role of the toxin in decreasing Samuel M. Chou, M.D., Ph.D. inhibitory input to alpha motoneurons is well known. Morphological alterations in neurons in Department of Pathology both clinical and experimental tetanus have not been consistently described. Indeed, several authors William N. Payne, M.D.* simply state that no changes are found. There are, however, reports of nuclear capping, and either chromophobia or chromophilia3 as well as central or perinuclear chromatolysis in motoneurons4,5 in tetanus. The finding of striking concurrence of cen- tral chromatolysis and vacuolation confined to mo- toneurons in a case of human tetanus prompts this report and a review of the literature. Case report A 58-year-old black man was admitted because of inability to swallow. The night before admission, he choked while trying to drink water; he also noted pain and stiffness in the neck and back. He was diabetic and took 40 units of insulin each morning. He had had an ulcer on his left heel for the preceding 10 months, but there was no history of recent wounds. He had not had a tetanus immunization for "many years." Chronic ulcers on the right leg had necessitated an above-the-knee am- putation l'/2 years before. The patient also had been * Section of Neuropathology, West Virginia taking phenytoin (Dilantin) and phenobarbital for a University Medical Center, Morgantown, West Virginia 26506. -

How Is the Brain Organized?
p CHAPTER 2 How Is the Brain Organized? An Overview of Brain Structure The Functional Organization Brain Terminology of the Brain The Brain’s Surface Features Principle 1: The Sequence of Brain Processing The Brain’s Internal Features Is “In Integrate Out” Microscopic Inspection: Cells and Fibers Principle 2: Sensory and Motor Divisions Exist Focus on Disorders: Meningitis and Throughout the Nervous System Encephalitis Principle 3: The Brain’s Circuits Are Crossed Focus on Disorders: Stroke Principle 4: The Brain Is Both Symmetrical and Asymmetrical Principle 5: The Nervous System Works A Closer Look at Neuroanatomy Through Excitation and Inhibition The Cranial Nervous System Principle 6: The Central Nervous System Has The Spinal Nervous System Multiple Levels of Function The Internal Nervous System Principle 7: Brain Systems Are Organized Both Focus on Disorders: Magendie, Bell, and Bell’s Hierarchically and in Parallel Palsy Principle 8: Functions in the Brain Are Both Localized and Distributed A. Klehr / Stone Images Micrograph: Carolina Biological Supply Co. / Phototake 36 I p hen buying a new car, people first inspect the In many ways, examining a brain for the first time is outside carefully, admiring the flawless finish similar to looking under the hood of a car. We have a vague W and perhaps even kicking the tires. Then they sense of what the brain does but no sense of how the parts open the hood and examine the engine, the part of the car that we see accomplish these tasks. We may not even be responsible for most of its behavior—and misbehavior. able to identify many of the parts.