Developmental Requirement of Gp130 Signaling in Neuronal Survival and Astrocyte Differentiation
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

CSF Protein Contents and Their Roles in Brain Development
Zahedan J Res Med Sci. 2015 September; 17(9):e1042. DOI: 10.17795/zjrms-1042 Review Article Published online 2015 September 26. CSF Protein Contents and Their Roles in Brain Development 1,* 1 1 Mohammad Nabiuni, Rozmehr Shokohi, and Parisa Moghaddam 1Department of Cell and Molecular Biology, Faculty of Biological Sciences, Kharazmi University, Tehran, IR Iran *Corresponding author : Mohammad Nabiuni, Department of Cell and Molecular Biology, Faculty of Biological Sciences, Kharazmi University, Tehran, IR Iran. E-mail: [email protected] Received: ; Accepted: January 6, 2014 March 18, 2014 Abstract: In early stages of development, the laminated structure of cerebral cortex is organized by proliferative, morphogenetic, and migratory processes. In these stages, cells within the ependymal lining of neural tube are thought to secrete embryonic cerebrospinal fluid (eCSF). As the neural tube closes, the choroid plexuses (CPs) secrete proteins such as growth factors, cytokines and morphogenes into the eCSF. The apical neuroepithelium is bathed with this fluid which plays regulatory roles in cortical cell proliferation, differentiation, and maintenance. Because of the eCSF protein contents and their impacts on neurogenesis, we focused on the effect of eCSF growth factors and their changes during brain development. Bibliographic databases including PubMed, Scopus and Google Scholar were searched between years 1990 to 2013 for the keywords “Cerebrospinal fluid” and “Neurogenesis”. In the first step, 200 articles were found, after elimination of duplicates or irrelevant papers 49 papers were selected and reviewed. Keywords: Cerebrospinal fluid; Cytokine; Neurogenesis; Choroid plexus; Cell differentiation 1. Context The central nervous system (CNS) develops from the bryogenesis. The CP diferentiates from the ependymal neural tube, a hollow structure filled with embryonic ce- cells lining the ventricular walls and, in fact, is frequent- rebrospinal fluid (eCSF) and surrounded by neuroepithe- ly considered to be a specialized cuboidal epithelium lial cells. -
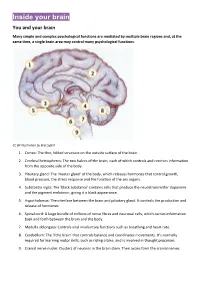
Inside Your Brain You and Your Brain
Inside your brain You and your brain Many simple and complex psychological functions are mediated by multiple brain regions and, at the same time, a single brain area may control many psychological functions. CC BY Illustration by Bret Syfert 1. Cortex: The thin, folded structure on the outside surface of the brain. 2. Cerebral hemispheres: The two halves of the brain, each of which controls and receives information from the opposite side of the body. 3. Pituitary gland: The ‘master gland’ of the body, which releases hormones that control growth, blood pressure, the stress response and the function of the sex organs. 4. Substantia nigra: The ‘black substance’ contains cells that produce the neurotransmitter dopamine and the pigment melatonin, giving it a black appearance. 5. Hypothalamus: The interface between the brain and pituitary gland. It controls the production and release of hormones. 6. Spinal cord: A large bundle of millions of nerve fibres and neuronal cells, which carries information back and forth between the brain and the body. 7. Medulla oblongata: Controls vital involuntary functions such as breathing and heart rate. 8. Cerebellum: The ‘little brain’ that controls balance and coordinates movements. It’s normally required for learning motor skills, such as riding a bike, and is involved in thought processes. 9. Cranial nerve nuclei: Clusters of neurons in the brain stem. Their axons form the cranial nerves. Your brain underpins who you are. It stores your knowledge and memories, gives you the capacity for thought and emotion, and enables you to control your body. The brain is just one part of the nervous system. -

The Genetic Basis of Mammalian Neurulation
REVIEWS THE GENETIC BASIS OF MAMMALIAN NEURULATION Andrew J. Copp*, Nicholas D. E. Greene* and Jennifer N. Murdoch‡ More than 80 mutant mouse genes disrupt neurulation and allow an in-depth analysis of the underlying developmental mechanisms. Although many of the genetic mutants have been studied in only rudimentary detail, several molecular pathways can already be identified as crucial for normal neurulation. These include the planar cell-polarity pathway, which is required for the initiation of neural tube closure, and the sonic hedgehog signalling pathway that regulates neural plate bending. Mutant mice also offer an opportunity to unravel the mechanisms by which folic acid prevents neural tube defects, and to develop new therapies for folate-resistant defects. 6 ECTODERM Neurulation is a fundamental event of embryogenesis distinct locations in the brain and spinal cord .By The outer of the three that culminates in the formation of the neural tube, contrast, the mechanisms that underlie the forma- embryonic (germ) layers that which is the precursor of the brain and spinal cord. A tion, elevation and fusion of the neural folds have gives rise to the entire central region of specialized dorsal ECTODERM, the neural plate, remained elusive. nervous system, plus other organs and embryonic develops bilateral neural folds at its junction with sur- An opportunity has now arisen for an incisive analy- structures. face (non-neural) ectoderm. These folds elevate, come sis of neurulation mechanisms using the growing battery into contact (appose) in the midline and fuse to create of genetically targeted and other mutant mouse strains NEURAL CREST the neural tube, which, thereafter, becomes covered by in which NTDs form part of the mutant phenotype7.At A migratory cell population that future epidermal ectoderm. -

Clonal Dispersion During Neural Tube Formation 4097 of Neuromeres
Development 126, 4095-4106 (1999) 4095 Printed in Great Britain © The Company of Biologists Limited 1999 DEV2458 Successive patterns of clonal cell dispersion in relation to neuromeric subdivision in the mouse neuroepithelium Luc Mathis1,*, Johan Sieur1, Octavian Voiculescu2, Patrick Charnay2 and Jean-François Nicolas1,‡ 1Unité de Biologie moléculaire du Développement, Institut Pasteur, 25, rue du Docteur Roux, 75724 Paris Cedex 15, France 2Unité INSERM 368, Ecole Normale Supérieure, 46 rue d’Ulm, 75230 Paris Cedex 05, France *Present address: Beckman Institute (139-74), California Institute of Technology, Pasadena, CA, 91125, USA ‡Author for correspondence (e-mail: [email protected]) Accepted 5 July; published on WWW 23 August 1999 SUMMARY We made use of the laacz procedure of single-cell labelling the AP and DV axis of the neural tube. A similar sequence to visualize clones labelled before neuromere formation, in of AP cell dispersion followed by an arrest of AP cell 12.5-day mouse embryos. This allowed us to deduce two dispersion, a preferential DV cell dispersion and then by a successive phases of cell dispersion in the formation of the coherent neuroepithelial growth, is also observed in the rhombencephalon: an initial anterior-posterior (AP) cell spinal cord and mesencephalon. This demonstrates that a dispersion, followed by an asymmetrical dorsoventral (DV) similar cascade of cell events occurs in these different cell distribution during which AP cell dispersion occurs in domains of the CNS. In the prosencephalon, differences in territories smaller than one rhombomere. We conclude that spatial constraints may explain the variability in the the general arrest of AP cell dispersion precedes the onset orientation of cell clusters. -

The GATA2 Transcription Factor Negatively Regulates the Proliferation of Neuronal Progenitors
RESEARCH ARTICLE 2155 Development 133, 2155-2165 (2006) doi:10.1242/dev.02377 The GATA2 transcription factor negatively regulates the proliferation of neuronal progenitors Abeer El Wakil*, Cédric Francius*,†, Annie Wolff, Jocelyne Pleau-Varet† and Jeannette Nardelli†,§ Postmitotic neurons are produced from a pool of cycling progenitors in an orderly fashion that requires proper spatial and temporal coordination of proliferation, fate determination, differentiation and morphogenesis. This probably relies on complex interplay between mechanisms that control cell cycle, specification and differentiation. In this respect, we have studied the possible implication of GATA2, a transcription factor that is involved in several neuronal specification pathways, in the control of the proliferation of neural progenitors in the embryonic spinal cord. Using gain- and loss-of-function manipulations, we have shown that Gata2 can drive neural progenitors out of the cycle and, to some extent, into differentiation. This correlates with the control of cyclin D1 transcription and of the expression of the p27/Kip1 protein. Interestingly, this functional aspect is not only associated with silencing of the Notch pathway but also appears to be independent of proneural function. Consistently, GATA2 also controls the proliferation capacity of mouse embryonic neuroepithelial cells in culture. Indeed, Gata2 inactivation enhances the proliferation rate in these cells. By contrast, GATA2 overexpression is sufficient to force such cells and neuroblastoma cells to stop dividing but not to drive either type of cell into differentiation. Furthermore, a non-cell autonomous effect of Gata2 expression was observed in vivo as well as in vitro. Hence, our data have provided evidence for the ability of Gata2 to inhibit the proliferation of neural progenitors, and they further suggest that, in this regard, Gata2 can operate independently of neuronal differentiation. -

Stem Cells and Neurological Disease the Transplant Site
J Neurol Neurosurg Psychiatry: first published as 10.1136/jnnp.74.5.553 on 1 May 2003. Downloaded from EDITORIAL 553 Stem cells shown to survive and ameliorate behav- ................................................................................... ioural deficits in an animal mode of Par- kinson’s disease,3 although in this study 20% of rats still developed teratomas at Stem cells and neurological disease the transplant site. In contrast, Kim et al, using a different approach that relies on R A Barker, M Jain,RJEArmstrong, M A Caldwell transfection with Nurr1 (a transcription ................................................................................... factor involved in the differentiation of dopaminergic cells), have demonstrated The therapeutic implications and application of stem cells for functional efficacy without tumour formation.4 the nervous system Human embryonic stem cells have now been isolated5 and grown in culture with enrichment for neuronal lineages, here has recently been a great deal of (c) ability to migrate and disseminate possible through exposure to a combina- interest in stem cells and the nerv- following implantation within the adult tion of growth factors and mitogens.6 Tous system, in terms of their poten- CNS; These cells, when placed in the develop- tial for deciphering developmental issues (d) possible tropism for areas of path- ing rat brain, can migrate widely and as well as their therapeutic potential. In ology; differentiate in a site specific fashion this editorial we will critically appraise without the formation of teratomas.7 the different types of stem cells, their (e) ease of manipulation using viral and non-viral gene transfer methods; However, the safety of these cells needs therapeutic implications, and the appli- further investigation before they can be (f) ability to better integrate into normal cations to which they have been put, considered for clinical use. -

Timing and Pattern of Cell Fate Restrictions in the Neural Crest Lineage
Development 124, 4351-4359 (1997) 4351 Printed in Great Britain © The Company of Biologists Limited 1997 DEV1236 Timing and pattern of cell fate restrictions in the neural crest lineage Paul D. Henion* and James A. Weston Institute of Neuroscience, University of Oregon, Eugene, OR 97403, USA *Present address: Neurobiotechnology Center and Department of Cell Biology, Neurobiology and Anatomy, Ohio State University, 105 Rightmire Hall, 1060 Carmack Road, Columbus, OH 43210, USA *Author for correspondence (e-mail: [email protected]) SUMMARY The trunk neural crest of vertebrate embryos is a transient sublineages were also present in the outgrowth population collection of precursor cells present along the dorsal aspect almost immediately, but melanogenic precursors dispersed of the neural tube. These cells migrate on two distinct from the neural tube only after many neurogenic precur- pathways and give rise to specific derivatives in precise sors had already done so. A discrete fate-restricted embryonic locations. One group of crest cells migrates neuronal precursor population was distinguished before early on a ventral pathway and generates neurons and glial entirely separate fate-restricted melanocyte and glial cells. A later-dispersing group migrates laterally and gives precursor populations were present, and well before initial rise to melanocytes in the skin. These observations raise the neuronal differentiation. Taken together, our results possibility that the appearance of distinct derivatives in demonstrate that lineage-restricted subpopulations consti- different embryonic locations is a consequence of lineage tute a major portion of the initial neural crest population restrictions specified before or soon after the onset of and that neural crest diversification occurs well before neural crest cell migration. -

Epidermal Growth Factor Promotes a Neural Phenotype in Thymic
Epidermal Growth Factor Promotes a Neural Phenotype in Thymic Epithelial Cells and Enhances Neuropoietic Cytoldne Expression Isabella Screpanti,* Susanna Scarpa,* Daniela Meco,* Diana BeUavia,~ Liborio Stuppia, § Luigi Frati, *u Andrea Modesti,* and Alberto Gulino I *Department of Experimental Medicine and Pathology, University La Sapienza, 00161 Rome;qnstitute of Human Pathology and Social Medicine, University of Chieti, 66100 Chieti; §Institute of N.P. Human Cytomorphology, National Research Council, 66100 Chieti; and UMediterranean Institute of Neuroscience, Pozzilli and IDepartment of Experimental Medicine, University of L'Aquila, 67100 L'Aquila, Italy Abstract. Neural crest-derived cells populate the thy- growth factor enhances cells that express the genes en- Downloaded from http://rupress.org/jcb/article-pdf/130/1/183/1264385/183.pdf by guest on 29 September 2021 mus, and their coexistence with epithelial cells is re- coding the preprotachykinin A-generated neuropep- quired for proper organ development and T cell educa- tides and the bipotential neuropoietic and lymphopoi- tion function. We show here that epidermal growth etic cytokines ciliary neurotrophic factor and factor (EGF), a major epithelial cell growth-enhancing interleukin-6. These cytokines also enhance the neu- agent, has a morphogenetic action to promote the ex- ronal phenotype of thymic epithelial cells. Therefore, pression of a neuronal phenotype (e.g., neurofilament EGF appears to be a composite autocrine/paracrine expression) in cultured thymic epithelial cells that are neuromodulator in thymic stroma. This suggests that characterized by a cytokeratin-positive epithelial cell EGF may regulate thymus-dependent immune func- background. The proliferation of such neurodifferenti- tions by promoting neuronal gene expression in neural ated cells is also enhanced by EGF. -

Homocysteine Intensifies Embryonic LIM3 Expression in Migratory Neural Crest Cells: a Quantitative Confocal Microscope Study
University of Northern Iowa UNI ScholarWorks Dissertations and Theses @ UNI Student Work 2014 Homocysteine intensifies embryonic LIM3 expression in migratory neural crest cells: A quantitative confocal microscope study Jordan Naumann University of Northern Iowa Let us know how access to this document benefits ouy Copyright ©2014 Jordan Naumann Follow this and additional works at: https://scholarworks.uni.edu/etd Part of the Biology Commons Recommended Citation Naumann, Jordan, "Homocysteine intensifies embryonic LIM3 expression in migratory neural crest cells: A quantitative confocal microscope study" (2014). Dissertations and Theses @ UNI. 89. https://scholarworks.uni.edu/etd/89 This Open Access Thesis is brought to you for free and open access by the Student Work at UNI ScholarWorks. It has been accepted for inclusion in Dissertations and Theses @ UNI by an authorized administrator of UNI ScholarWorks. For more information, please contact [email protected]. Copyright by JORDAN NAUMANN 2014 All Rights Reserved HOMOCYSTEINE INTENSIFIES EMBRYONIC LIM3 EXPRESSION IN MIGRATORY NEURAL CREST CELLS – A QUANTITATIVE CONFOCAL MICROSCOPE STUDY An Abstract of a Thesis Submitted in Partial Fulfillment of the Requirements for the Degree Master of Science Jordan Naumann University of Northern Iowa May 2014 ABSTRACT Elevated levels of homocysteine in maternal blood and amniotic fluid are associated with cardiovascular, renal, skeletal, and endocrine diseases and also with embryonic malformations related to neural crest cells. Neural crest cells are necessary for the formation of tissues and organs throughout the body of vertebrate animals. The migration of neural crest cells is essential for proper development of the target tissues. When migration is disrupted, abnormalities may occur. -

Microglia and Macrophages in the Pathological Central and Peripheral Nervous Systems
cells Review Microglia and Macrophages in the Pathological Central and Peripheral Nervous Systems Naoki Abe 1, Tasuku Nishihara 1,*, Toshihiro Yorozuya 1 and Junya Tanaka 2 1 Department of Anesthesia and Perioperative Medicine, Ehime University Graduate School of Medicine, Toon, Ehime 791-0295, Japan; [email protected] (N.A.); [email protected] (T.Y.) 2 Department of Molecular and cellular Physiology, Ehime University Graduate School of Medicine, Toon, Ehime 791-0295, Japan; [email protected] * Correspondence: [email protected]; Tel.: +81-89-960-5383; Fax: +81-89-960-5386 Received: 4 August 2020; Accepted: 17 September 2020; Published: 21 September 2020 Abstract: Microglia, the immunocompetent cells in the central nervous system (CNS), have long been studied as pathologically deteriorating players in various CNS diseases. However, microglia exert ameliorating neuroprotective effects, which prompted us to reconsider their roles in CNS and peripheral nervous system (PNS) pathophysiology. Moreover, recent findings showed that microglia play critical roles even in the healthy CNS. The microglial functions that normally contribute to the maintenance of homeostasis in the CNS are modified by other cells, such as astrocytes and infiltrated myeloid cells; thus, the microglial actions on neurons are extremely complex. For a deeper understanding of the pathophysiology of various diseases, including those of the PNS, it is important to understand microglial functioning. In this review, we discuss both the favorable and unfavorable roles of microglia in neuronal survival in various CNS and PNS disorders. We also discuss the roles of blood-borne macrophages in the pathogenesis of CNS and PNS injuries because they cooperatively modify the pathological processes of resident microglia. -

Gamma Motor Neurons Survive and Exacerbate Alpha Motor Neuron Degeneration In
Gamma motor neurons survive and exacerbate alpha PNAS PLUS motor neuron degeneration in ALS Melanie Lalancette-Heberta,b, Aarti Sharmaa,b, Alexander K. Lyashchenkoa,b, and Neil A. Shneidera,b,1 aCenter for Motor Neuron Biology and Disease, Columbia University, New York, NY 10032; and bDepartment of Neurology, Columbia University, New York, NY 10032 Edited by Rob Brownstone, University College London, London, United Kingdom, and accepted by Editorial Board Member Fred H. Gage October 27, 2016 (received for review April 4, 2016) The molecular and cellular basis of selective motor neuron (MN) the muscle spindle and control the sensitivity of spindle afferent vulnerability in amyotrophic lateral sclerosis (ALS) is not known. In discharge (15); beta (β) skeletofusimotor neurons innervate both genetically distinct mouse models of familial ALS expressing intra- and extrafusal muscle (16). In addition to morphological mutant superoxide dismutase-1 (SOD1), TAR DNA-binding protein differences, distinct muscle targets, and the absence of primary 43 (TDP-43), and fused in sarcoma (FUS), we demonstrate selective afferent (IA)inputsonγ-MNs, these functional MN subtypes also degeneration of alpha MNs (α-MNs) and complete sparing of differ in their trophic requirements, and γ-MNs express high levels gamma MNs (γ-MNs), which selectively innervate muscle spindles. of the glial cell line-derived neurotropic factor (GDNF) receptor Resistant γ-MNs are distinct from vulnerable α-MNs in that they Gfrα1 (17). γ-MNs are also molecularly distinguished by the ex- lack synaptic contacts from primary afferent (IA) fibers. Elimination pression of other selective markers including the transcription α of these synapses protects -MNs in the SOD1 mutant, implicating factor Err3 (18), Wnt7A (19), the serotonin receptor 1d (5-ht1d) this excitatory input in MN degeneration. -

Cadherins in Brain Compartmentalisation 563 Juxtaposing the Transmembrane Domain of Pca-Cad6-HA Was Similarly Constructed
Development 128, 561-569 (2001) 561 Printed in Great Britain © The Company of Biologists Limited 2001 DEV1642 Role of cadherins in maintaining the compartment boundary between the cortex and striatum during development Takayoshi Inoue1,2, Taro Tanaka3, Masatoshi Takeichi3, Osamu Chisaka3, Shun Nakamura1 and Noriko Osumi4,* 1Division of Biochemistry and Cellular Biology, National Institute of Neuroscience, National Center of Neurology and Psychiatry, 4-1-1, Ogawahigashi, Kodaira, Tokyo 187-8502, Japan 2Japan Science and Technology Corporation, 4-1-8, Hon-machi, Kawaguchi, Saitama 332-0012, Japan 3Department of Biophysics, Faculty of Science, Kyoto University, Kitashirakawa, Sakyo-ku, Kyoto 606-8502, Japan 4Department of Developmental Neurobiology, Tohoku University Graduate School of Medicine, 2-1, Seiryo-machi, Aoba-ku, Sendai 980-8575, Japan *Author for correspondence (e-mail: [email protected]) Accepted 29 November 2000; published on WWW 23 January 2001 SUMMARY In ventricular cells of the mouse telencephalon, differential trend for ectopic R-cadherin-expressing striatal cells. expression of cadherin cell adhesion molecules defines Although cadherin-6 gene knockout mice engineered in neighbouring regions; R-cadherin delineates the future this study showed no obvious phenotype in telencephalic cerebral cortex, while cadherin-6 delineates the lateral compartmentalisation, the preferential sorting of ectopic ganglionic eminence. By using cell labelling analyses in the cadherin-6-expressing cells was abolished in this mutant whole embryo culture system, we demonstrated that the background. Thus, the differential expression pattern of interface between R-cadherin and cadherin-6 expression is cadherins in the embryonic telencephalon is responsible for a boundary for cell lineage restriction at embryonic day maintaining the cortico-striatal compartment boundary.