Alteration of Plant Physiology by Glyphosate and Its By-Product Aminomethylphosphonic Acid: an Overview
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

2,4-Dichlorophenoxyacetic Acid
2,4-Dichlorophenoxyacetic acid 2,4-Dichlorophenoxyacetic acid IUPAC (2,4-dichlorophenoxy)acetic acid name 2,4-D Other hedonal names trinoxol Identifiers CAS [94-75-7] number SMILES OC(COC1=CC=C(Cl)C=C1Cl)=O ChemSpider 1441 ID Properties Molecular C H Cl O formula 8 6 2 3 Molar mass 221.04 g mol−1 Appearance white to yellow powder Melting point 140.5 °C (413.5 K) Boiling 160 °C (0.4 mm Hg) point Solubility in 900 mg/L (25 °C) water Related compounds Related 2,4,5-T, Dichlorprop compounds Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) 2,4-Dichlorophenoxyacetic acid (2,4-D) is a common systemic herbicide used in the control of broadleaf weeds. It is the most widely used herbicide in the world, and the third most commonly used in North America.[1] 2,4-D is also an important synthetic auxin, often used in laboratories for plant research and as a supplement in plant cell culture media such as MS medium. History 2,4-D was developed during World War II by a British team at Rothamsted Experimental Station, under the leadership of Judah Hirsch Quastel, aiming to increase crop yields for a nation at war.[citation needed] When it was commercially released in 1946, it became the first successful selective herbicide and allowed for greatly enhanced weed control in wheat, maize (corn), rice, and similar cereal grass crop, because it only kills dicots, leaving behind monocots. Mechanism of herbicide action 2,4-D is a synthetic auxin, which is a class of plant growth regulators. -

Delayed Fluorescence Imaging of Photosynthesis Inhibitor and Heavy Metal Induced Stress in Potato
Cent. Eur. J. Biol. • 7(3) • 2012 • 531-541 DOI: 10.2478/s11535-012-0038-z Central European Journal of Biology Delayed fluorescence imaging of photosynthesis inhibitor and heavy metal induced stress in potato Research Article Jaka Razinger1,*, Luka Drinovec2, Maja Berden-Zrimec3 1Agricultural Institute of Slovenia, 1000 Ljubljana, Slovenia 2Aerosol d.o.o., 1000 Ljubljana, Slovenia 3Institute of Physical Biology, 1000 Ljubljana, Slovenia Received 09 November 2011; Accepted 13 March 2012 Abstract: Early chemical-induced stress in Solanum tuberosum leaves was visualized using delayed fluorescence (DF) imaging. The ability to detect spatially heterogeneous responses of plant leaves exposed to several toxicants using delayed fluorescence was compared to prompt fluorescence (PF) imaging and the standard maximum fluorescence yield of PSII measurements (Fv/Fm). The toxicants used in the study were two photosynthesis inhibitors (herbicides), 100 μM methyl viologen (MV) and 140 μM diuron (DCMU), and two heavy metals, 100 μM cadmium and 100 μM copper. The exposure times were 5 and 72 h. Significant photosynthesis-inhibitor effects were already visualized after 5 h. In addition, a significant reduction in the DF/PF index was measured in DCMU- and MV-treated leaves after 5 h. In contrast, only DCMU-treated leaves exhibited a significant decrease in Fv/Fm after 5 h. All treatments resulted in a significant decrease in the DF/PF parameter after 72 h of exposure, when only MV and Cd treatment resulted in visible symptoms. Our study highlights the power of delayed fluorescence imaging. Abundant quantifiable spatial information was obtained with the instrumental setup. Delayed fluorescence imaging has been confirmed as a very responsive and useful technique for detecting stress induced by photosynthesis inhibitors or heavy metals. -

Evidence for Different Binding Sites on the 33-Kda Protein for DCMU, Atrazine and QB
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Elsevier - Publisher Connector Volume 167, number 2 FEBS 1259 February 1984 Evidence for different binding sites on the 33-kDa protein for DCMU, atrazine and QB Chantal Astier, Alain Boussac and Anne-Lise Etienne Laboratoire de PhotosynthPse, CNRS, BP I, 91190 Gifsur-Yvette, France Received 21 November 1983; revised version received 4 January 1984 Two DCMU-resistant strains of the cyanobacterium Synechocystis 6714 were used to analyse the binding sites of DCMU, atrazine and QB. DCMU’-11~was DCMU and atrazine resistant; it presented an impaired electron flow and its 33-kDa protein was weakly attached to the membrane. DCMU’-IIB, derived from the former, simultaneously regained atrazine sensitivity, normal electron flow and a tight linkage of the 33-kDa protein to the membrane. This mutant shows that loss of DCMU binding does not necessarily affect the binding of either atrazine or QB. The role of the 33-kDa protein is discussed. Cyanobacteria Mutant Herbicide Photosynthesis Photosystem ZZ 1. INTRODUCTION that a specific attack of lysine residues resulted also in a partial release of inhibition by SN58132, Electron transfer on the acceptor side of a DCMU-type inhibitor [17]. Photosystem II occurs through a primary acceptor Radioactivity labelling experiments were used to QA [l] (a particular plastoquinone of the general demonstrate a competition between DCMU and pool [2] closely associated with the chlorophyll atrazine [6,18-201. A similar competition was pro- center), a secondary acceptor Qn [3] bound to its posed in [l l] between DCMU and QB. -

Interactions of Paraquat and Nitrodiphenylether Herbicides with the Chloroplast Photosynthetic Electron Transport in the Activation of Toxic Oxygen Species
INTERACTIONS OF PARAQUAT AND NITRODIPHENYLETHER HERBICIDES WITH THE CHLOROPLAST PHOTOSYNTHETIC ELECTRON TRANSPORT IN THE ACTIVATION OF TOXIC OXYGEN SPECIES by Brad Luther Upham Dissertation submitted to the Faculty of the Virginia Polytechnic Institute and State University in partial fulfillment of the requirements for the degree of Doctor of Philosophy in Plant Physiology APPROVED: Kriton K. Hatzios, Chairman -----..------,,...-r---~---------J ohn L. Hess Joyce G. Foster Laurence D. Moore David M. Orcutt William E. Winner INTERACTIONS OF PARAQUAT AND.NITRODIPHENYLETHER HERBICIDES WITH THE PHOTOSYNTHETIC ELECTRON TRANSPORT IN THE ACTIVATION OF TOXIC OXYGEN SPECIES by Brad Luther Upham Kriton K. Hatzios, Chairman Plant Physiology (ABSTRACT) The interactions of paraquat (methylviologen) and diphenylether her- bicides with the Mehler reaction as investigated. Sera from two differ- ent rabbits (RSl & RS2) were examined for their patterns of inhibition of the photosynthetic electron transport (PET) system. Serum from RS2 was greatly hemolyzed. Fifty ul of RSI serum were required for 100% inhibi- tion of a 1120 --> methylviologen(MV)/02 reaction, whereas only 10 µl of a 1:10 dilution of RS2 were needed for 100% inhibition. The ~-globulin fraction from purified rabbit serum (RSl) did not inhibit PET, indicat- ing that the antibody fraction of the rabbit serum does not contain the inhibitor. It appears that the inhibitor is from the hemolyzed red blood cells. Rabbit sera, added to chloroplast preparations prior illu- mination, caused no inhibition of a 1120 --> MV/02 reaction while addi- tion of rabbit sera during illumination inhibited the 1120 --> MV/02 reaction within 1-3 s. Various Hill reactions were used to determine the site of inhibition. -

DCMU) Or Paraquat-Treated Euglena Gracilis Cells Erich F
Chlorophyll Photobleaching and Ethane Production in Dichlorophenyldimethylurea- (DCMU) or Paraquat-Treated Euglena gracilis Cells Erich F. Elstner and W. Osswald Institut für Botanik und Mikrobiologie, Technische Universität München, Arcisstr. 21, D-8000 München 2 Z. Naturforsch. 35 c, 129-135 (1980); received August 20/September 28, 1979 Chlorophyll Bleaching, Herbicides, Euglena gracilis, Ethane, Fat Oxidation Light dependent (35 Klux) chlorophyll bleaching in autotrophically grown Euglena gracilis cells at slightly acidic pH (6.5 —5.4) is stimulated by the photosystem II blockers DCMU and DBMIB (both 10~ 5 m) as well as by the autooxidizable photosystem I electron acceptor, paraquat (1 0 -3 m ). Chlorophyll photobleaching is accompanied by the formation of thiobarbituric acid — sensitive material (“malondialdehyde”) and ethane. Both chlorophyll photobleaching and light dependent ethane formation are partially prevented by higher concentrations (10~* m ) of the autooxidizable photosystem II electron acceptor DBMIB or by sodium bicarbonate (25 mM). In vitro studies with cell free extracts (homogenates) from E. gracilis suggest that a-linolenic acid oxidation by excited (reaction center II) chlorophyll represents the driving force for both ethane formation and chlorophyll bleaching. Ethane formation thus appears to be a sensitive and non-destructive “in vivo’’ marker for both restricted energy dissipation in photosystem II and, conditions yielding reactive oxygen species at the reducing side o f photosystem I. Introduction quent lipid attack and membrane destruction in the case of low potential photosystem I electron ac Chlorophyll bleaching is one of the characteristic ceptors as paraquat and other bipyridylium salts symptoms for plant diseases introduced by infec [4, 5, 9], tions, certain physical parameters or by chemicals. -
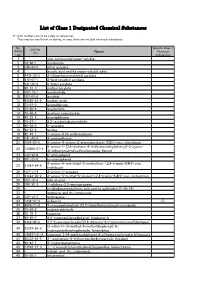
List of Class 1 Designated Chemical Substances
List of Class 1 Designated Chemical Substances *1:CAS numbers are to be solely as references. They may be insufficient or lacking, in case there are multiple chemical substances. No. Specific Class 1 CAS No. (PRTR Chemical (*1) Name Law) Substances 1 - zinc compounds(water-soluble) 2 79-06-1 acrylamide 3 140-88-5 ethyl acrylate 4 - acrylic acid and its water-soluble salts 5 2439-35-2 2-(dimethylamino)ethyl acrylate 6 818-61-1 2-hydroxyethyl acrylate 7 141-32-2 n-butyl acrylate 8 96-33-3 methyl acrylate 9 107-13-1 acrylonitrile 10 107-02-8 acrolein 11 26628-22-8 sodium azide 12 75-07-0 acetaldehyde 13 75-05-8 acetonitrile 14 75-86-5 acetone cyanohydrin 15 83-32-9 acenaphthene 16 78-67-1 2,2'-azobisisobutyronitrile 17 90-04-0 o-anisidine 18 62-53-3 aniline 19 82-45-1 1-amino-9,10-anthraquinone 20 141-43-5 2-aminoethanol 21 1698-60-8 5-amino-4-chloro-2-phenylpyridazin-3(2H)-one; chloridazon 5-amino-1-[2,6-dichloro-4-(trifluoromethyl)phenyl]-3-cyano- 22 120068-37-3 4[(trifluoromethyl)sulfinyl]pyrazole; fipronil 23 123-30-8 p-aminophenol 24 591-27-5 m-aminophenol 4-amino-6-tert-butyl-3-methylthio-1,2,4-triazin-5(4H)-one; 25 21087-64-9 metribuzin 26 107-11-9 3-amino-1-propene 27 41394-05-2 4-amino-3-methyl-6-phenyl-1,2,4-triazin-5(4H)-one; metamitron 28 107-18-6 allyl alcohol 29 106-92-3 1-allyloxy-2,3-epoxypropane 30 - n-alkylbenzenesulfonic acid and its salts(alkyl C=10-14) 31 - antimony and its compounds 32 120-12-7 anthracene 33 1332-21-4 asbestos ○ 34 4098-71-9 3-isocyanatomethyl-3,5,5-trimethylcyclohexyl isocyanate 35 78-84-2 isobutyraldehyde -

20210311 IAEG AD-DSL V5.0 for Pdf.Xlsx
IAEGTM AD-DSL Release Version 4.1 12-30-2020 Authority: IAEG Identity: AD-DSL Version number: 4.1 Issue Date: 2020-12-30 Key Yellow shading indicates AD-DSL family group entries, which can be expanded to display a non-exhaustive list of secondary CAS numbers belonging to the family group Substance Identification Change Log IAEG Regulatory Date First Parent Group IAEG ID CAS EC Name Synonyms Revision Date ECHA ID Entry Type Criteria Added IAEG ID IAEG000001 1327-53-3 215-481-4 Diarsenic trioxide Arsenic trioxide R1;R2;D1 2015-03-17 2015-03-17 100.014.075 Substance Direct Entry IAEG000002 1303-28-2 215-116-9 Diarsenic pentaoxide Arsenic pentoxide; Arsenic oxide R1;R2;D1 2015-03-17 2015-03-17 100.013.743 Substance Direct Entry IAEG000003 15606-95-8 427-700-2 Triethyl arsenate R1;R2;D1 2015-03-17 2017-08-14 100.102.611 Substance Direct Entry IAEG000004 7778-39-4 231-901-9 Arsenic acid R1;R2;D1 2015-03-17 2015-03-17 100.029.001 Substance Direct Entry IAEG000005 3687-31-8 222-979-5 Trilead diarsenate R1;R2;D1 2015-03-17 2017-08-14 100.020.890 Substance Direct Entry IAEG000006 7778-44-1 231-904-5 Calcium arsenate R1;R2;D1 2015-03-17 2017-08-14 100.029.003 Substance Direct Entry IAEG000009 12006-15-4 234-484-1 Cadmium arsenide Tricadmium diarsenide R1;R2;D1 2017-08-14 2017-08-14 Substance Direct Entry IAEG000021 7440-41-7 231-150-7 Beryllium (Be) R2 2015-03-17 2019-01-24 Substance Direct Entry IAEG000022 1306-19-0 215-146-2 Cadmium oxide R1;R2;D1 2015-03-17 2017-08-14 100.013.770 Substance Direct Entry IAEG000023 10108-64-2 233-296-7 Cadmium -

(12) United States Patent (10) Patent No.: US 6,190,773 B1 Imamura Et Al
USOO6190773B1 (12) United States Patent (10) Patent No.: US 6,190,773 B1 Imamura et al. (45) Date of Patent: Feb. 20, 2001 (54) SELF-WATER DISPERSIBLE PARTICLE Primary Examiner Samuel A. Acquah MADE OF BIODEGRADABLE POLYESTER (74) Attorney, Agent, or Firm-Armstrong, Westerman, AND PROCESS FOR THE PREPARATION Hattori, McLeland & Naughton THEREOF (57) ABSTRACT (75) Inventors: Shoji Imamura, Sakura; Yasuyuki Watanabe, Chiba; Kazuaki Tsukuda; A Self-water dispersible particle made of a biodegradable Takashi Hirokawa, both of Sakura; polyester and a process for the preparation of an aqueous Nagao Ariga, Abiko, all of (JP) dispersion of Self-water dispersible particles made of a (73) Assignee: Dainippon Ink and Chemicals, Inc., biodegradable polyester containing a hydrophobic core Tokyo (JP) material, which comprises reacting a biodegradable polyes ter containing hydroxyl group with a polyvalent carboxylic (*) Notice: Under 35 U.S.C. 154(b), the term of this acid or anhydride or chloride thereof, dissolving or dispers patent shall be extended for 0 days. ing the biodegradable polyester having acid groups thus obtained and a hydrophobic core material in an organic (21) Appl. No.: 09/296,132 Solvent, adding a base to the Solution with Stirring to (22) Filed: Apr. 22, 1999 neutralize to form the Salt of the biodegradable polyester, and then adding water to the Solution or dispersion to (30) Foreign Application Priority Data undergo phase inversion emulsification are disclosed. Apr. 23, 1998 (JP) ................................................. 10-113343 According to the present invention, a Self-water dispersible Mar. 25, 1999 (JP) ................................................. 11-081359 particle made of a biodegradable polyester having varied (51) Int. -

(Bio)Sensors for Pesticides Detection Using Screen-Printed Electrodes
biosensors Review Electrochemical (Bio)Sensors for Pesticides Detection Using Screen-Printed Electrodes Beatriz Pérez-Fernández , Agustín Costa-García y and Alfredo de la Escosura- Muñiz * NanoBioAnalysis Group-Department of Physical and Analytical Chemistry, University of Oviedo, Julián Clavería 8, 33006 Oviedo, Spain * Correspondence: [email protected]; Tel.: +34-985-103-521 In Memoriam to Prof. Agustín Costa-García. y Received: 12 March 2020; Accepted: 30 March 2020; Published: 2 April 2020 Abstract: Pesticides are among the most important contaminants in food, leading to important global health problems. While conventional techniques such as high-performance liquid chromatography (HPLC) and mass spectrometry (MS) have traditionally been utilized for the detection of such food contaminants, they are relatively expensive, time-consuming and labor intensive, limiting their use for point-of-care (POC) applications. Electrochemical (bio)sensors are emerging devices meeting such expectations, since they represent reliable, simple, cheap, portable, selective and easy to use analytical tools that can be used outside the laboratories by non-specialized personnel. Screen-printed electrodes (SPEs) stand out from the variety of transducers used in electrochemical (bio)sensing because of their small size, high integration, low cost and ability to measure in few microliters of sample. In this context, in this review article, we summarize and discuss about the use of SPEs as analytical tools in the development of (bio)sensors for pesticides of interest for food control. Finally, aspects related to the analytical performance of the developed (bio)sensors together with prospects for future improvements are discussed. Keywords: screen-printed electrodes; electrochemical (bio)sensors; pesticides; point-of-care; food control 1. -

Emerging Strategies for the Bioremediation of the Phenylurea Herbicide Diuron
fmicb-12-686509 August 6, 2021 Time: 15:23 # 1 REVIEW published: 12 August 2021 doi: 10.3389/fmicb.2021.686509 Emerging Strategies for the Bioremediation of the Phenylurea Herbicide Diuron Jiayi Li1,2, Wenping Zhang1,2, Ziqiu Lin1,2, Yaohua Huang1,2, Pankaj Bhatt1,2 and Shaohua Chen1,2* 1 State Key Laboratory for Conservation and Utilization of Subtropical Agro-Bioresources, Guangdong Province Key Laboratory of Microbial Signals and Disease Control, Integrative Microbiology Research Centre, South China Agricultural University, Guangzhou, China, 2 Guangdong Laboratory for Lingnan Modern Agriculture, Guangzhou, China Diuron (DUR) is a phenylurea herbicide widely used for the effective control of most annual and perennial weeds in farming areas. The extensive use of DUR has led to its widespread presence in soil, sediment, and aquatic environments, which poses a threat to non-target crops, animals, humans, and ecosystems. Therefore, the removal of DUR from contaminated environments has been a hot topic for researchers in recent decades. Bioremediation seldom leaves harmful intermediate metabolites and is emerging as the most effective and eco-friendly strategy for removing DUR from Edited by: the environment. Microorganisms, such as bacteria, fungi, and actinomycetes, can Xingxing Peng, Sun Yat-sen University, China use DUR as their sole source of carbon. Some of them have been isolated, including Reviewed by: organisms from the bacterial genera Arthrobacter, Bacillus, Vagococcus, Burkholderia, Fabrice Martin-Laurent, Micrococcus, Stenotrophomonas, and Pseudomonas and fungal genera Aspergillus, Institut National de la Recherche Pycnoporus, Pluteus, Trametes, Neurospora, Cunninghamella, and Mortierella. A Agronomique (INRA), France Alessandro D’Annibale, number of studies have investigated the toxicity and fate of DUR, its degradation Tuscia University, Italy pathways and metabolites, and DUR-degrading hydrolases and related genes. -

Effect of Biochar Amendment and Ageing on Adsorption and Degradation of Two Herbicides
Water Air Soil Pollut (2017) 228:216 DOI 10.1007/s11270-017-3392-7 Effect of Biochar Amendment and Ageing on Adsorption and Degradation of Two Herbicides Alena Zhelezova & Harald Cederlund & John Stenström Received: 25 November 2016 /Accepted: 10 May 2017 # The Author(s) 2017. This article is an open access publication Abstract Biochar amendment can alter soil properties, a soil historically enriched with charcoal. Biochar for instance, the ability to adsorb and degrade different amendment increased the pH in both soils and increased chemicals. However, ageing of the biochar, due to pro- the water-holding capacity of the sandy soil. Adsorption cesses occurring in the soil over time, can influence such of diuron was enhanced by biochar amendment in both biochar-mediated effects. This study examined how soils, while glyphosate adsorption was decreased in the biochar affected adsorption and degradation of two her- sandy soil. Ageing of soil-biochar mixtures decreased bicides, glyphosate (N-(phosphonomethyl)-glycine) and adsorption of both herbicides in comparison with freshly diuron (3-(3,4-dichlorophenyl)-1,1-dimethylurea) in biochar-amended soil. Herbicide degradation rates were soil and how these effects were modulated by ageing not consistently affected by biochar amendment or age- of the biochar. One sandy and one clayey soil that had ing in any of the soils. However, glyphosate half-lives been freshly amended with a wood-based biochar (0, 1, correlated with the Freundlich Kf values in the clayey 10, 20 and 30% w/w) were studied. An ageing experi- soil, indicating that degradation was limited by avail- ment, in which the soil-biochar mixtures were aged for ability there. -

Studies on the Varietal Susceptibility in Winter
STUDIES ON THE VARIETAL SUSCEPTIBILITY IN WINTER WHEAT TO SUBSTITUTED PHENYLUREA HERBICIDES A Thesis submitted by REZA EMAMI-SARAVI a candidate for the degree of Doctor of Philosophy in BIOCHEMISTRY Department of Biochemistry Royal Holloway College University of London Egham Hill Egham January 1979 Surrey ProQuest Number: 10097467 All rights reserved INFORMATION TO ALL USERS The quality of this reproduction is dependent upon the quality of the copy submitted. In the unlikely event that the author did not send a complete manuscript and there are missing pages, these will be noted. Also, if material had to be removed, a note will indicate the deletion. uest. ProQuest 10097467 Published by ProQuest LLC(2016). Copyright of the Dissertation is held by the Author. All rights reserved. This work is protected against unauthorized copying under Title 17, United States Code. Microform Edition © ProQuest LLC. ProQuest LLC 789 East Eisenhower Parkway P.O. Box 1346 Ann Arbor, Ml 48106-1346 (i) ACKN OWLEDGEMENTS My sincere thanks and gratitude must go to my supervisor. Dr. W.J. Owen, for introducing me to such an interesting topic, for his help, encouragement, and patience throughout my research, and for his guidance in the preparation of this thesis. Secondly, I wish to thank Professor J.B. Pridham and Dr. W.A. Stevens for their interest, advice and helpful discussions throughout the duration of this work. My gratitude is also extended to all other members of staff and to students of this College who have helped me either practically or theoretically during my postgraduate research and in the preparation of this thesis.