Nuttall Grad.Sunysb 0771M 10261
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Long Island South Shore Estuary Reserve Coordinated Water Resources Monitoring Strategy Long Island SOUTH SHORE ESTUARY RESERVE
Long Island South Shore Estuary Reserve Coordinated Water Resources Monitoring Strategy New York Suffolk Nassau Long Island SOUTH SHORE ESTUARY RESERVE Open-File Report 2017–1161 U.S. Department of the Interior U.S. Geological Survey Cover. The Long Island South Shore Estuary Reserve (orange) stretches west to east from the Nassau-Queens county line to the town of Southampton. South to north, it extends from mean high tide on the ocean side of the barrier islands to the inland limits of the watersheds that drain into the bays. Image courtesy of the New York State Department of State Office of Planning, Development and Community Infrastructure. Long Island South Shore Estuary Reserve Coordinated Water Resources Monitoring Strategy By Shawn C. Fisher, Robert J. Welk, and Jason S. Finkelstein Prepared in cooperation with the New York State Department of State Office of Planning, Development and Community Infrastructure and the South Shore Estuary Reserve Office Open-File Report 2017–1161 U.S. Department of the Interior U.S. Geological Survey U.S. Department of the Interior RYAN K. ZINKE, Secretary U.S. Geological Survey James F. Reilly II, Director U.S. Geological Survey, Reston, Virginia: 2018 For more information on the USGS—the Federal source for science about the Earth, its natural and living resources, natural hazards, and the environment—visit https://www.usgs.gov or call 1–888–ASK–USGS. For an overview of USGS information products, including maps, imagery, and publications, visit https://store.usgs.gov. Any use of trade, firm, or product names is for descriptive purposes only and does not imply endorsement by the U.S. -

Carmans, Patchogue, and Swan Rivers
Estuaries and Coasts: J CERF DOI 10.1007/s12237-007-9010-y Temporal and Spatial Variations in Water Quality on New York South Shore Estuary Tributaries: Carmans, Patchogue, and Swan Rivers Lori Zaikowski & Kevin T. McDonnell & Robert F. Rockwell & Fred Rispoli Received: 9 April 2007 /Revised: 23 August 2007 /Accepted: 14 September 2007 # Coastal and Estuarine Research Federation 2007 Abstract The chemical and biological impacts of anthro- magnitude higher than SR and CR sediments. Benthic pogenic physical modifications (i.e., channelization, dredg- invertebrate assessment of species richness, biotic index, ing, bulkhead, and jetty construction) to tributaries were and Ephemeroptera, Plecoptera and Trichoptera richness assessed on New York’s Long Island South Shore Estuary. indicated that PR was severely impacted, SR ranged from Water-quality data collected on Carmans, Patchogue, and slightly to severely impacted, and CR ranged from non- Swan Rivers from 1997 to 2005 indicate no significant impacted to slightly impacted. Diversity and abundance of differences in nutrient levels, temperature, or pH among the plankton were comparable on SR and CR, and were rivers, but significant differences in light transmittance, significantly higher than on PR. The data indicate that dissolved oxygen (DO), salinity, and sediments were nutrients do not play a major role in hypoxia in these observed. Patchogue River (PR) and Swan River (SR) were estuarine tributaries but that physical forces dominate. The significantly more saline than Carmans River (CR), PR and narrow inlets, channelization, and abrupt changes in depth SR had less light transmittance than CR, and both exhibited near the inlets of PR and SR foster hypoxic conditions by severe warm season hypoxia. -

Revised Draft Subwatersheds Wastewater Plan Executive Summary
REVISED DRAFT SUBWATERSHEDS WASTEWATER PLAN EXECUTIVE SUMMARY “We are in a county that will no longer allow our water quality crisis to go unaddressed, but will come together to Reclaim Our Water” Suffolk County Executive Steve Bellone 2014 State of the County Suffolk County Department of Health Services August 2019 This document was prepared with funding provided by the New York State Department of Environmental Conservation as part of the Long Island Nitrogen Action Plan and by New York State Department of State under the Environmental Protection Fund. Subwatersheds Wastewater Plan • Executive Summary Table of Contents Executive Summary ......................................................................................................... 5 1.0 Introduction ................................................................................................................................................................. 5 1.1 Background ........................................................................................................................................................ 5 1.2 Wastewater Management in Suffolk County ........................................................................................ 7 1.3 Innovative/Alternative Onsite Wastewater Treatment Systems ................................................ 9 1.4 Sewage Treatment Plants and Sewering ............................................................................................ 13 1.4.1 Sewer Expansion Projects .......................................................................................................... -

WHOI-R-93-006 Anderson, D.M. an Immunof
J ournal of Plankton Research Vo1.15 no.5 pp.563-580, 1993 An immunofluorescent survey of the brown tide chrysophyte A ureococcus anophagefferens along the northeast coast of the United States Donald M.Anderson, Bruce A.Keafer, David M.Kulis, Robert M.Waters 1 and Robert Nuzzi 1 Woods Hole Oceanographic Institution, Biology Department, Woods Hole, MA 02543 and 1 Suffolk County Department of Health Services, Evans K. Griffing County Center, Riverhead, NY JJ 901, USA Abst ract. Surveys were conducted along the northeast coast of the USA, be1ween Po rtsmouth, NH, and I he Chesapeake Bay in 1988 and 1990, to determine the population distribulion of Aureococcus anoplwgefferens, lhe chrysophyte responsible for massive and destructive 'brown tides' in Long Island and Narraganselt Bay beginning in 1985. A species-specific immunofluorescent technique was used to screen water samples, with positive identification possible at cell concentrations as low as 10- 1 20 cells ml - • Bolh years, A.anophagefferens was detected at numerous stations in and around Long Island and Barnegat Bay, J , typically at high cell concentrations. To the north and south of this 'center·, nearly half of the remaining stations were positive for A.anophagefferens, but the cells were always at very low cell concentrations. Many of the positive identifications in areas distant from Long Island were in waters with no known history of harmful brown tides. The species was present in both open coastal and estuarine locations, in salinities between 18 and 32 prac1ical salinity units (PSU). The observed population distributions apparently still reflect the massive 1985 outbreak when this species first bloomed, given the number of positive locations and high abundance of A.anophagef ferens in the immediate vicinity of Long Island. -
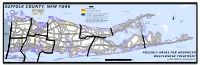
Surface Waters Contributing Areas
Fishers Island Sound Legend SUFFOLK COUNTY, NEW YORK Suffolk County Land Use PriorityUnsewered - 1* Residential*** Huntington Long Island Sound Bay PriorityUnsewered - 2* Residential*** Lloyd Harbor Rd Long Island Sound Baseflowyear 0-2 Con tributingAreas to SurfaceWaters, d r R Main Rd e 1000’arounbuffer freshwater d lakes andpon ds iv R w Cold Northport Bay ro ar Spring W N N E Shore Rd e Baseflowyear 2-25 Con tributingAreas to SurfaceWaters North Rd c Harbor k Main St R Orient Harbor d Smithtown Bay * Priority 1 on this map is depicted by medium and high density residential parcels falling either partially or entirely within the 0-2 Year Baseflow Front St o e Main Rd Av d ** Priority 2 on this map is depicted by medium and high density residential parcels ew Mill Pond R Main St vi nga d Block Fort S alo n falling either partially or entirely within the 2-25 Year Baseflow u ÂC25A Main St C25A Port o A Â Jefferson S 25 Great Pond Island te *** Unsewered Residential on this map is depicted by medium density residential N ou F SHELTER R e 5 N r e 2 r (>1 to < 5 du/acre) and high density residential (>or equal to 5 du/acre) t o Sound u y o s t r R ÂC108 Old Dock Rd R a d Woodbury Rd Hallock Ave n d Route 25A d N Ferry Rd ISLAND ulaski R Bridge Ln P P k E Main St w Gardiners y Brander Pkwy e v A ÂCSM Oregon Rd Cox Ln M Bay k Route 112 r a N Bayview Rd i S Ferry Rd o n Y Ba d Miller Place Yaphank Rd SOUTHOLD yv w i R e Rd Menantic e y W Jericho Tpk ntr w e N u Miller Place Rd Mill Ln R HUNTINGTON o Canal Rd Route 25A d SMITHTOWN C ÂC25A ÂC25 -

Village of Patchogue Revitalization, Economic Impact Analysis Prepared For: Long Island Regional Planning Council
Village of Patchogue Revitalization, Economic Impact Analysis Prepared for: Long Island Regional Planning Council ECONOMIC AND REAL ESTATE ANALYSIS FOR SUSTAINABLE LAND USE OUTCOMES™ [ProjectVillage of Name] Patchogue Economic and Fiscal Impact Analysis Study December 19, 2018 Table of Contents Executive Summary 4 Background Review 21 A Brief History of Patchogue’s Economic Rise, Fall & Rise 23 Timeline of Public & Private Investments in Patchogue 28 Interviews with Public & Private Sector Representatives 38 Economic Impact Analysis 48 Development Construction 53 New Non-Local Household Spending 59 New Business Operations 66 Fiscal Impacts 79 Multi-Family Housing Development Projects Built Since 2004 80 Prospective Large-Scale Development Projects 95 Documentation of the Increase in Assessed Property Values 114 Comparative Business, Employment & Investment Trends 118 Appendix 129 4WARD PLANNING LLCINC Page 2 [ProjectVillage of Name] Patchogue Economic and Fiscal Impact Analysis Study December 19, 2018 Acknowledgements The Long Island Regional Planning Council acknowledges and appreciates the support of the Suffolk County Economic Development Corporation in producing this Study. 4ward Planning wishes to thank the following individuals for providing invaluable insights and for, particularly, helping to facilitate the study process over a period of several months: Mayor Paul Pontieri Marian H. Russo, Executive Director, Patchogue Community Development Agency (CDA) David Kennedy, Executive Director, Village of Patchogue Chamber of Commerce We also thank the Town of Brookhaven for its generous assistance with identifying critical property tax and revenue data. 4WARD PLANNING LLCINC Page 3 Executive Summary ECONOMIC AND REAL ESTATE ANALYSIS FOR SUSTAINABLE LAND USE OUTCOMES ™ [ProjectVillage of Name] Patchogue Economic and Fiscal Impact Analysis Study December 19, 2018 Introduction The redevelopment of the Village of Patchogue has been one of Long Island’s foremost community improvement success stories. -

Long Island Duck Farm History and Ecosystem Restoration Opportunities Suffolk County, Long Island, New York
Long Island Duck Farm History and Ecosystem Restoration Opportunities Suffolk County, Long Island, New York February 2009 US Army Corps of Engineers Suffolk County, NY New York District Table of Contents Section Page Table of Contents............................................................................................................................ 1 List of Appendices .......................................................................................................................... 1 1.0 Introduction.............................................................................................................................. 1 2.0 Purpose..................................................................................................................................... 1 3.0 History of Duck Farming on Long Island................................................................................ 1 4.0 Environmental Impacts ............................................................................................................. 2 4.1 Duck Waste Statistics ....................................................................................................... 2 4.2 Off-site Impacts of Duck Farm Operation........................................................................ 3 4.2.1 Duck Sludge Deposits.................................................................................................... 4 4.3 On-site Impacts of Duck Farm Operation......................................................................... 5 5.0 -

Suffolk County Subwatersheds
SUFFOLK COUNTY SUBWATERSHEDS WASTEWATER PLAN NYSDEC •LINAP LINAP •SCUPE/Funding USEPA •NYSDEC •LISS •LIRPC Coordinate •Technical • •PMT Experts • Align •LICAP • Consistency… NYSDOS SBU •SSER •SoMAS •WPAC •Funding Partner •CCWT Advisory Reclaim Our Water Estuaries Committees/ •PEP Technical Project Partners •LISS Experts* •SSER *Consists of Suffolk County Wastewater Plan •County Executive Advisory Committee, Stakeholders •Legislature Article 5/6 workgroup, •NGOs (too many to •DEDP wastewater plan focus list!) Towns/ •DHS area workgroups, and Villages •DPW technical experts (SBU, •Parks USGS, SCWA, Cornell Coop, etc.) SUBWATERSHEDS WASTEWATER PLAN Science Based Bridge to Support Policy Decisions - Transition from Septic Demo and SIP to wide-scale implementation Provide recommended blueprint for wastewater upgrades: Set priority areas, nitrogen load reduction goals, and describe where, when, and what methods to implement to meet reduction goals (I/A OWTS, sewering, clustered, other) Establish uniform and consistent set of subwatershed boundaries for all priority areas (surface water, drinking water, groundwater) Develop nitrogen load rates Develop receiving water residence times (surface water sensitivity) Establish baseline water quality Establishment of tiered priority areas Define endpoints (e.g., water clarity, dissolved oxygen, HABs, SAV, etc.) Establish first order nitrogen load reduction goals for all of the County’s surface water, drinking water, and groundwater resources Recommendations for wastewater upgrades for each -

Cruising Guide of the SOUTH BAY CRUISING CLUB
Cruising Guide of the SOUTH BAY CRUISING CLUB This Cruising Guide is intended to be a practical handbook to assist members of the South Bay Cruising Club (SBCC) sail the Great South Bay and the waters that the SBCC summer cruisers roam: providing the skipper with information. When used in conjunction with the latest Nautical Charts, Coast Pilot, Light List, Tide and Current Publication and a copy of the Rules of the Road (boats larger than 39 feet must have a copy aboard) will provide the ship’s captain and crew with an enjoyable cruising season. 1 ©2017 by South Bay Cruising Club 2016-2017 Edition Prologue ruising. …Blue sky and dancing seas beckon, exciting voyages, the peace and C loveliness of quiet anchorages, camaraderie and the majestic crimson sunset await the cruiser, at night fall when the sails are furled and the ensign has been lowered one can relax on deck and study the heavens. Since the original edition (1966) and two revised publications, many changes have taken place. This, the 2016 edition, continues the tradition of providing local information to SBCC members now includes New England, Connecticut, New York, New Jersey, Delaware and Maryland. Care has been taken to provide the SBCC membership with accurate information. Nevertheless, remember that as time goes by buoy locations change, telephone numbers and area codes change, marinas close and new ones open; inlet waterways shift. Do your homework before setting sail on a journey. The committee suggests that members use this copy as the name implies as a guide and not for navigation. -

Brown Tide Session Growth of Aureococcus Anophagefferens on Complex Sources of Dissolved Organic Nitrogen in Culture
ABSTRACTS OF ORAL PRESENTATIONS BROWN TIDE SESSION GROWTH OF AUREOCOCCUS ANOPHAGEFFERENS ON COMPLEX SOURCES OF DISSOLVED ORGANIC NITROGEN IN CULTURE Gry Mine Berg1, Julie LaRoche1, Dan Repeta2 1Institut für Meereskunde, Kiel University, 24105 Kiel, Germany 2Woods Hole Oceanographic Institution, Woods Hole, MA 02540, USA Aureococcus anophagefferens repeatedly blooms in several Long Island (New York, USA) embayments, forming "brown tides" that discolor the water. Surveys of the northeast coast of the USA have shown that A. anophagefferens exits in several places that have no records of brown tide. Therefore, the recurrence of the brown tide in Long Island is somewhat unusual. The coastal bays in Long Island are strongly influenced by groundwater, contributing the largest input of fixed nitrogen. In years of draught and low - groundwater flow, the supply of NO3 is sharply reduced leaving dissolved organic nitrogen (DON) as the largest source of nitrogen available to the phytoplankton. The ability of A. anophagefferens to grow on DON has been hypothesized to be an important factor in sustaining the brown tide during periods of dissolved inorganic nitrogen depletion. In order to test this hypothesis we prepared an axenic culture of A. anophagefferens and followed growth with a number of DON substrates as the sole source of nitrogen in culture. In addition to commercially available substrates we used >1 kDa ultrafiltered DON isolated from West Neck Bay (WNB) pore waters, Long Island. Efforts to characterize components of the bulk DON pool were conducted in parallel with investigations of the bioavailability of these components. For the preparation of an axenic, artificial seawater culture of strain CCMP 1784 we modified a protocol published by Cottrell and Suttle (1995 J. -

Ken Schultz's Field Guide to Saltwater Fish
ffirs.qxd 10/31/03 9:25 AM Page iii • • • • KEN SCHULTZ’S Field Guide to Saltwater Fish by Ken Schultz JOHN WILEY & SONS, INC. ffirs.qxd 10/31/03 9:25 AM Page i • • • • KEN SCHULTZ’S Field Guide to Saltwater Fish ffirs.qxd 10/31/03 9:25 AM Page ii ffirs.qxd 10/31/03 9:25 AM Page iii • • • • KEN SCHULTZ’S Field Guide to Saltwater Fish by Ken Schultz JOHN WILEY & SONS, INC. ffirs.qxd 10/31/03 9:25 AM Page iv This book is printed on acid-free paper. Copyright © 2004 by Ken Schultz. All rights reserved Published by John WIley & Sons, Inc., Hoboken, New Jersey Published simultaneously in Canada Design and production by Navta Associates, Inc. No part of this publication may be reproduced, stored in a retrieval system, or trans- mitted in any form or by any means, electronic, mechanical, photocopying, recording, scanning, or otherwise, except as permitted under Section 107 or 108 of the 1976 United States Copyright Act, without either the prior written permission of the Pub- lisher, or authorization through payment of the appropriate per-copy fee to the Copy- right Clearance Center, 222 Rosewood Drive, Danvers, MA 01923, (978) 750-8400, fax (978) 750-4470, or on the web at www.copyright.com. Requests to the Publisher for permission should be addressed to the Permissions Department, John Wiley & Sons, Inc., 111 River Street, Hoboken, NJ 07030, (201) 748-6011, fax (201) 748-6008, email: [email protected]. Limit of Liability/Disclaimer of Warranty: While the publisher and the author have used their best efforts in preparing this book, they make no representations or warranties with respect to the accuracy or completeness of the contents of this book and specifi- cally disclaim any implied warranties of merchantability or fitness for a particular purpose. -

Contribution of Waterborne Nitrogen Emissions to Hypoxia-Driven Marine Eutrophication: Modelling of Damage to Ecosystems in Life Cycle Impact Assessment (LCIA)
Downloaded from orbit.dtu.dk on: Mar 30, 2019 Contribution of waterborne nitrogen emissions to hypoxia-driven marine eutrophication: modelling of damage to ecosystems in life cycle impact assessment (LCIA) Cosme, Nuno Miguel Dias Publication date: 2016 Document Version Publisher's PDF, also known as Version of record Link back to DTU Orbit Citation (APA): Cosme, N. M. D. (2016). Contribution of waterborne nitrogen emissions to hypoxia-driven marine eutrophication: modelling of damage to ecosystems in life cycle impact assessment (LCIA). Technical University of Denmark (DTU). General rights Copyright and moral rights for the publications made accessible in the public portal are retained by the authors and/or other copyright owners and it is a condition of accessing publications that users recognise and abide by the legal requirements associated with these rights. Users may download and print one copy of any publication from the public portal for the purpose of private study or research. You may not further distribute the material or use it for any profit-making activity or commercial gain You may freely distribute the URL identifying the publication in the public portal If you believe that this document breaches copyright please contact us providing details, and we will remove access to the work immediately and investigate your claim. Contribution of waterborne nitrogen emissions to hypoxia-driven marine eutrophication: modelling of damage to ecosystems in life cycle impact assessment (LCIA) Nuno Miguel Dias Cosme PhD Thesis May 2016 Division for Quantitative Sustainability Assessment Department of Management Engineering Technical University of Denmark Cosme N. 2016. Contribution of waterborne nitrogen emissions to hypoxia-driven marine eutrophication: modelling of damage to ecosystems in life cycle impact assessment (LCIA).