Journal of Ginseng Research
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Report on Domestic Animal Genetic Resources in China
Country Report for the Preparation of the First Report on the State of the World’s Animal Genetic Resources Report on Domestic Animal Genetic Resources in China June 2003 Beijing CONTENTS Executive Summary Biological diversity is the basis for the existence and development of human society and has aroused the increasing great attention of international society. In June 1992, more than 150 countries including China had jointly signed the "Pact of Biological Diversity". Domestic animal genetic resources are an important component of biological diversity, precious resources formed through long-term evolution, and also the closest and most direct part of relation with human beings. Therefore, in order to realize a sustainable, stable and high-efficient animal production, it is of great significance to meet even higher demand for animal and poultry product varieties and quality by human society, strengthen conservation, and effective, rational and sustainable utilization of animal and poultry genetic resources. The "Report on Domestic Animal Genetic Resources in China" (hereinafter referred to as the "Report") was compiled in accordance with the requirements of the "World Status of Animal Genetic Resource " compiled by the FAO. The Ministry of Agriculture" (MOA) has attached great importance to the compilation of the Report, organized nearly 20 experts from administrative, technical extension, research institutes and universities to participate in the compilation team. In 1999, the first meeting of the compilation staff members had been held in the National Animal Husbandry and Veterinary Service, discussed on the compilation outline and division of labor in the Report compilation, and smoothly fulfilled the tasks to each of the compilers. -

ICAES 2008 Yunnan Zhuang Language Use 26 April Final
The Southern Zhuang Languages of Yunnan Province’s Wenshan Prefecture from a Sociolinguistic Perspective 从社会语言学方面看 云南省文山州的壮语南部方言 Eric C. Johnson 江子扬 Susanne J. Johnson 马艾华 SIL International 世界少数民族语文研究院 East Asia Group 东亚部 2008 Eric C. Johnson and Susanne J. Johnson The Southern Zhuang Languages of Yunnan Province’s Wenshan Prefecture from a Sociolinguistic Perspective Abstract Although the majority of China’s 16 million Zhuang nationality people live in the Guangxi Zhuang Autonomous Region, over one million Zhuang also live in Yunnan Province, mostly in the Wenshan Zhuang and Miao Autonomous Prefecture in the extreme southeast of the province. More than half of these Zhuang speak Central Taic languages collectively known to linguists as “Southern Zhuang,” but referred to by their speakers as “Nong,” “Dai,” and “Min.” The goal of this paper is to introduce the sociolinguistic situation of the Yunnan Southern Zhuang languages, especially focusing on the current language use situation in rural Southern Zhuang villages, the present vitality of these languages and their prospects for future preservation and development. 1 Introduction The Zhuang people are the largest minority nationality within the People’s Republic of China, numbered at 16 million during the 2000 national census (National Bureau of Statistics 2003). Of these, approximately one third speak "Southern Zhuang," that is, Central Taic varieties, whereas two thirds speak "Northern Zhuang," or Northern Taic varieties. Over 1.1 million Zhuang nationality people live in Yunnan Province, and more than half of these are speakers of Central Taic language varieties. Though the Tai family as a whole has been the subject of a significant amount of research in Thailand, China, and elsewhere during the past century, much remains to be done. -

Download Article
Advances in Economics, Business and Management Research, volume 71 4th International Conference on Economics, Management, Law and Education (EMLE 2018) The Impact of Cross-border Migrant Employment on Workers' Income in Border Areas An Empirical Study Based on the Micro Data of Yunnan Border Areas Jie Yang Renjuan Luo School of Economics and Management School of Economics and Management Yunnan Normal University Yunnan Normal University Kunming, China Kunming, China Abstract—Based on household survey data of the national China, and "The Belt and Road" strategic planning. These social science fund research group in Yunnan border area, this regional development policies have provided new opportunities paper examines the marginal effects of cross-border mobile for the development of border areas. Population, as the main employment on the wage level of workers. We use the census body of regional economic and social development, has register's household register and whether there is someone going become an important indicator of regional development, abroad for business or working in the same region as instrument especially the trend and situation of floating employment of the variables to eliminate the endogeneity of cross-border flows working population, which is the focus of governments at all which lead to empirical bias. The study found that the cross- levels. In recent years, the employment pattern of population border flow of employment increased theirs wages significantly. flow in border areas has taken on new characteristics. In Even by controlling for factors of age, gender, education, national addition to the traditional inter-provincial flow, intra-provincial and income level before the outflow, the result is still significant and robust. -

The Occurrence of Pinus Massoniana Lambert (Pinaceae) from the Upper Miocene of Yunnan, SW China and Its Implications for Paleogeography and Paleoclimate
Review of Palaeobotany and Palynology 215 (2015) 57–67 Contents lists available at ScienceDirect Review of Palaeobotany and Palynology journal homepage: www.elsevier.com/locate/revpalbo The occurrence of Pinus massoniana Lambert (Pinaceae) from the upper Miocene of Yunnan, SW China and its implications for paleogeography and paleoclimate Jian-Wei Zhang a,AshalataD'Rozariob,JonathanM.Adamsc, Xiao-Qing Liang a, Frédéric M.B. Jacques a, Tao Su a, Zhe-Kun Zhou a,⁎ a Key Laboratory of Tropical Forest Ecology, Xishuangbanna Tropical Botanical Garden (XTBG), Chinese Academy of Sciences, Mengla, Yunnan 666303, China b Department of Botany, Narasinha Dutt College, 129, Bellilious Road, Howrah 711101, India c The college of Natural Sciences, Seoul National University, 1 Gwanak-ro, Gwanak-gu, Seoul 151-742, Republic of Korea article info abstract Article history: A fossil seed cone and associated needles from the upper Miocene Wenshan flora, Yunnan Province, SW China are Received 11 August 2014 recognized as Pinus massoniana Lambert, which is an endemic conifer distributed mostly in southern, central and Received in revised form 12 November 2014 eastern parts of China. The comparisons of these fossils with the three extant variants in this species Accepted 15 November 2014 (P. massoniana var. shaxianensis Zhou, P. massoniana var. massoniana Lambert and P. massoniana var. hainanensis Available online 15 December 2014 Cheng et Fu) indicate that the fossils closely resemble P. massoniana var. hainanensis, which is a tropical montane thermophilic and hygrophilous plant restricted to Hainan Island in southern China. The present finding and a pre- Keywords: fi China vious report of Pinus premassoniana from the same age in southeastern China, which bears close af nities with Comparative morphology modern P. -

Table of Codes for Each Court of Each Level
Table of Codes for Each Court of Each Level Corresponding Type Chinese Court Region Court Name Administrative Name Code Code Area Supreme People’s Court 最高人民法院 最高法 Higher People's Court of 北京市高级人民 Beijing 京 110000 1 Beijing Municipality 法院 Municipality No. 1 Intermediate People's 北京市第一中级 京 01 2 Court of Beijing Municipality 人民法院 Shijingshan Shijingshan District People’s 北京市石景山区 京 0107 110107 District of Beijing 1 Court of Beijing Municipality 人民法院 Municipality Haidian District of Haidian District People’s 北京市海淀区人 京 0108 110108 Beijing 1 Court of Beijing Municipality 民法院 Municipality Mentougou Mentougou District People’s 北京市门头沟区 京 0109 110109 District of Beijing 1 Court of Beijing Municipality 人民法院 Municipality Changping Changping District People’s 北京市昌平区人 京 0114 110114 District of Beijing 1 Court of Beijing Municipality 民法院 Municipality Yanqing County People’s 延庆县人民法院 京 0229 110229 Yanqing County 1 Court No. 2 Intermediate People's 北京市第二中级 京 02 2 Court of Beijing Municipality 人民法院 Dongcheng Dongcheng District People’s 北京市东城区人 京 0101 110101 District of Beijing 1 Court of Beijing Municipality 民法院 Municipality Xicheng District Xicheng District People’s 北京市西城区人 京 0102 110102 of Beijing 1 Court of Beijing Municipality 民法院 Municipality Fengtai District of Fengtai District People’s 北京市丰台区人 京 0106 110106 Beijing 1 Court of Beijing Municipality 民法院 Municipality 1 Fangshan District Fangshan District People’s 北京市房山区人 京 0111 110111 of Beijing 1 Court of Beijing Municipality 民法院 Municipality Daxing District of Daxing District People’s 北京市大兴区人 京 0115 -

Yunnan Provincial Highway Bureau
IPP740 REV World Bank-financed Yunnan Highway Assets management Project Public Disclosure Authorized Ethnic Minority Development Plan of the Yunnan Highway Assets Management Project Public Disclosure Authorized Public Disclosure Authorized Yunnan Provincial Highway Bureau July 2014 Public Disclosure Authorized EMDP of the Yunnan Highway Assets management Project Summary of the EMDP A. Introduction 1. According to the Feasibility Study Report and RF, the Project involves neither land acquisition nor house demolition, and involves temporary land occupation only. This report aims to strengthen the development of ethnic minorities in the project area, and includes mitigation and benefit enhancing measures, and funding sources. The project area involves a number of ethnic minorities, including Yi, Hani and Lisu. B. Socioeconomic profile of ethnic minorities 2. Poverty and income: The Project involves 16 cities/prefectures in Yunnan Province. In 2013, there were 6.61 million poor population in Yunnan Province, which accounting for 17.54% of total population. In 2013, the per capita net income of rural residents in Yunnan Province was 6,141 yuan. 3. Gender Heads of households are usually men, reflecting the superior status of men. Both men and women do farm work, where men usually do more physically demanding farm work, such as fertilization, cultivation, pesticide application, watering, harvesting and transport, while women usually do housework or less physically demanding farm work, such as washing clothes, cooking, taking care of old people and children, feeding livestock, and field management. In Lijiang and Dali, Bai and Naxi women also do physically demanding labor, which is related to ethnic customs. Means of production are usually purchased by men, while daily necessities usually by women. -
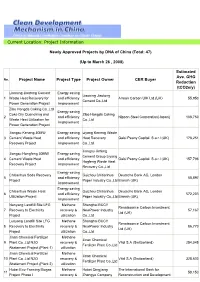
(Up to March 26 , 2008) Project Name P
Current Location: Project Information Newly Approved Projects by DNA of China (Total: 47) (Up to March 26 , 2008) Estimated Ave. GHG No. Project Name Project Type Project Owner CER Buyer Reduction (tCO2e/y) Liaoning Jiaotong Cement Energy saving Liaoning Jiaotong 1 Waste Heat Recovery for and efficiency Arreon Carbon UIK Ltd.(UK) 55,950 Cement Co.,Ltd Power Generation Project improvement Zibo Hongda Coking Co.,Ltd Energy saving Coke Dry Quenching and Zibo Hongda Coking 2 and efficiency Nippon Steel Corporation(Japan) 108,750 Waste Heat Utilization for Co.,Ltd improvement Power Generation Project Jiangsu Keneng 30MW Energy saving Liyang Keneng Waste 3 Cement Waste Heat and efficiency Heat Recovery Gaisi Peony Capital. S.a.r.l (UK) 178,251 Recovery Project improvement Co.,Ltd Jiangsu Jinfeng Jiangsu Hongfeng 30MW Energy saving Cement Group Liyang 4 Cement Waste Heat and efficiency Gaisi Peony Capital. S.a.r.l (UK) 157,796 Hogfeng Waste Heat Recovery Project improvement Recovery Co.,Ltd Energy saving Chitianhua Soda Recovery Guizhou Chitianhua Deutsche Bank AG, London 5 and efficiency 58,897 Project Paper Industry Co.,Ltd Branch (UK) improvement Energy saving Chitianhua Waste Heat Guizhou Chitianhua Deutsche Bank AG, London 6 and efficiency 572,203 Utilization Project Paper Industry Co.,Ltd Branch (UK) improvement Nanyang Landfill Site LFG Methane Shanghai BCCY Renaissance Carbon Investment 7 Recovery to Electricity recovery & NewPower Industry 57,167 Ltd (UK) Project utilization Co.,Ltd Luoyang Landfill Site LFG Methane Shanghai BCCY Renaissance -

2015-5-263.Pdf
(4) Schmidtiphaea yunnanensis Davies et Yang, 1996 05.31, number 0059052–0059064 and 0059066 is 1992.05.24 Schmidtiphaea yunnanensis Davies et Yang, 1996 (Davies & on label (all of them are 1993.05.24/31 in original description). Yang, 1996). Type locality: Jiangcheng County, Yunnan Province, China. Holotype: number 0059002. Paratypes: 3 specimens, number 0059003–0059005 (number 0059003 was allotype in II. ISOPTERA original description). Type locality: Jiangcheng County, Yunnan Province, China. (i) Rhinotermitidae (11) Heterotermes coelceps Zhu, Huang et Wang, 1992 (ii) Gomphidae Heterotermes coelceps Zhu, Huang et Wang, 1992 (Zhu et al, (5) Anisogomphus nitidus Yang et Davies, 1993 1992). Anisogomphus nitidus Yang et Davies, 1993 (Yang & Davies, Syntypes: 23 specimens, number 0060275–0060297. 1993). Authors didn’t indicate the holotype. Holotype: number 0059006. Type locality: Qianjiang County, Chongqing City (Sichuan The collector of number 0059006 is Allen and Davies on label Province), China. (DALD in original description). (12) Heterotermes dayongensis Zhu, Huang et Wang, 1992 Type locality: Dali, Yunnan Province, China. Heterotermes dayongensis Zhu, Huang et Wang, 1992 (Zhu (6) Anisogomphus resortus Yang et Davies, 1996 et al, 1992). Anisogomphus resortus Yang et Davies, 1996 (Yang & Syntypes: 55 specimens, number 0060062–0060116. Davies, 1996). Authors didn’t indicate the holotype. Holotype: number 0059040. Paratype: 1 specimen, number Type locality: Zhangjiajie National Park, Dayong City, Hunan 0059041. Province, China. The collecting dates of number 0059040 and 0059041 are (13) Heterotermes leigongshanensis Zhu, Huang, Wang et 1993.06.10 and 1992.06.08 on label respectively (1993.06.08 Han, 1992 and 1992.07.10 in original description). Heterotermes leigongshanensis Zhu, Huang, Wang et Han, Type locality: Emeishan Mountain, Sichuan Province, China. -

China - Provisions of Administration on Border Trade of Small Amount and Foreign Economic and Technical Cooperation of Border Regions, 1996
China - Provisions of Administration on Border Trade of Small Amount and Foreign Economic and Technical Cooperation of Border Regions, 1996 MOFTEC copy @ lexmercatoria.org Copyright © 1996 MOFTEC SiSU lexmercatoria.org ii Contents Contents Provisions of Administration on Border Trade of Small Amount and Foreign Eco- nomic and Technical Cooperation of Border Regions (Promulgated by the Ministry of Foreign Trade Economic Cooperation and the Customs General Administration on March 29, 1996) 1 Chapter 1 - General Provisions 1 Article 1 ......................................... 1 Article 2 ......................................... 1 Article 3 ......................................... 1 Chapter 2 - Border Trade of Small Amount 1 Article 4 ......................................... 1 Article 5 ......................................... 2 Article 6 ......................................... 2 Article 7 ......................................... 2 Article 8 ......................................... 3 Article 9 ......................................... 3 Article 10 ........................................ 3 Article 11 ........................................ 3 Article 12 ........................................ 4 Article 13 ........................................ 4 Article 14 ........................................ 4 Article 15 ........................................ 4 Article 16 ........................................ 5 Article 17 ........................................ 5 Chapter 3 - Foreign Economic and Technical Cooperation in Border Regions -

Cultural Revolution on the Border: Yunnan's 'Political Frontier Defence' (1969-1971)
Cultural Revolution on the Border: Cultural Revolution on the Border: Yunnan's 'Political Frontier Defence' (1969-1971) MICHAEL SCHOENHALS Abstract This paper addresses an important but so far neglected episode in the post- 1949 history of China – the impact of the so-called 'Cultural Revolution' on the country's ethnic minority populations. Specifically, it attempts to deal with the movement as it unfolded in the province of Yunnan where, at one stage, it be- came an attempt by a political leadership in the provincial capital, dominated by military officers and supported by members of the central authorities in Beijing, to alter the landscape of the ethnic minority populations along the frontier. Using information culled from local histories and contemporary sources, the pa- per traces the history of what even by the standards of the Cultural Revolution (1966-76) came to be regarded as an exceptionally flawed and counterproductive policy. It foregrounds the human cost of its implementation and, for the first time, goes some way towards explaining – in more than simply general terms of labels like 'excesses' and 'ultra-leftism' – the trauma of those who survived it, a trauma that to this day still lingers in popular memory.1 Introduction During the 'Great Proletarian Cultural Revolution', China's southern frontier – where the province of Yunnan borders on Vietnam, Laos and Burma – witnessed the implementation of something called a 'political frontier defence' (zhengzhi bianfang) (PFD). As part of the Cultural Revo- lution, the PFD has left no traces in the works of historians concerned with national-level events and developments, nor has it figured in social science scholarship concerned with the organization of political power and its consequences in the 'Maoist system' (Barnouin and Yu 1993; MacFarquhar and Fairbank 1991; Yan and Gao 1996). -

Cenozoic Magmatic Activity and Oblique Uplifting of the Ailao
SCIENCE CHINA Earth Sciences •RESEARCH PAPER• September 2020 Vol.63 No.9: 1294–1308 https://doi.org/10.1007/s11430-019-9616-y Cenozoic magmatic activity and oblique uplifting of the Ailao Mountain: Evidence from a short-period dense seismic array Lu ZHANG1,2, Zhiming BAI1,3*, Tao XU1,4, Zhenbo WU5, Minfu HUANG1,2, Guiping YU1,2, Junlei CHEN1,2,6 & Mengjie ZHENG1,2 1 State Key Laboratory of Lithospheric Evolution, Institute of Geology and Geophysics, Chinese Academy of Sciences, Beijing 100029, China; 2 University of Chinese Academy of Sciences, Beijing 100049, China; 3 Innovation Academy for Earth Science, Chinese Academy of Sciences, Beijing 100029, China; 4 CAS Center for Excellence in Tibetan Plateau Earth Sciences, Beijing 100101, China; 5 School of Geophysics, Chengdu University of Techonology, Chengdu 610059, China; 6 Earthquake Administration of Yunnan Province, Kunming 650224, China Received December 15, 2019; revised April 13, 2020; accepted April 20, 2020; published online May 25, 2020 Abstract Geological studies show that the southern part of Ailaoshan-Red River shear zone (ALSRRSZ) has experienced complex metallogenic processes and multi-stage non-uniform uplifting, called oblique uplifting since the Cenozoic. To detect the deep structure and geodynamic background beneath Daping, Chang’an and other gold and polymetallic deposits in this area, we carried out a high-density short-period seismic array survey in southern Yunnan province. The array used is approximately 240 km long with an interval of 500 m between two adjacent stations. Based on the data collected by the array, we used H-κ stacking and common conversion point (CCP) methods of receiver functions to reveal the fine crustal structure beneath this array, which was located from Lvchun (western end) to the east and ended in Maguan. -

World Bank Document
IPP252 World Bank Financed Yunnan Urban Environmental Project Public Disclosure Authorized SOCIAL ASSESSMENT: INDIGENOUS PEOPLES Public Disclosure Authorized Public Disclosure Authorized China Cross-cultural Consulting Center, Sun Yat-sen University July 13, 2007 Public Disclosure Authorized Yunan Province –World Bank 1 Group Huit –SCE YUEP-fasep706 Summary According to the OP4.10, TOR provided by SA expert of the WB, and under the guidance of DRA SA consultant, during Dec 1-19, 2006, the social assessment experts of the China Cross-cultural Consulting Center, Sun Yat-sen University (CCCC) conducted a field survey to identify the features, social impacts, benefit mechanism on the indigenous peoples (IPs) involved in the 34 subprojects under the World Bank Financed Yunnan Urban Environmental Project (YUEP). The Yunnan Urban Environmental Construction Project covers 86 Xiangs/towns/sub-districts in 22 counties/districts in Kunming, Dali, Lijiang and Wenshan Prefectures/Cities, Yunnan Province, with a total benefited population of 2.13million, including an urban population of 1.16 million (54.4%) and a rural population of 0.97 million (45.6%). The negatively impacted population is 11,1321(accounting for 0.52% of beneficiaries ) , including 3,667 in Kuming project area, 2,815 in Dali project area, 2,417 in Lijiang Project area, and 2,233 in Wenshan project area. The affected regions include 12 key counties for state poverty relief, including five IPs’ autonomous counties and two IPs' autonomous prefectures, 15 counties in total, namely Shilin Yi Autonomous County, Luquan Miao Autonomous County, Xundian Hui-Yi Autonomous County, Ninglang Naxi-Yi Autonomous County, Yulong Naxi Autonomous County, Dali City, Eryuan County, Funing County, Malipo County, Guangnan County, Yanshan County, Wenshan County, Qiubei County, Xichou County and Maguan County.