Bacteriology
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Meningitis Manual Text
Laboratory Methods for the Diagnosis of MENINGITIS Caused by Neisseria meningitidis, Streptococcus pneumoniae, and Haemophilus influenzae Centers for Disease Control and Prevention August, 1998 Laboratory Methods for the Diagnosis of Meningitis Caused by Neisseria meningitidis, Streptococcus pneumoniae, and Haemophilus influenzae Table of Contents Introduction………………………………………………………………………………… 1 Acknowledgments ……………………………………………………………………….. 2 I. Epidemiology of Meningitis Caused by Neisseria meningitidis, Haemophilus influenzae and Streptococcus pneumoniae,…………………………………………… 3 II. General Considerations ......................................................................................................... 5 A. Record Keeping ................................................................................................................... 5 III. Collection and Transport of Clinical Specimens ................................................................... 6 A. Collection of Cerebrospinal Fluid (CSF)............................................................................... 6 A1. Lumbar Puncture ................................................................................................... 6 B. Collection of Blood .............................................................................................................. 7 B1. Precautions ............................................................................................................ 7 B2. Sensitivity of Blood Cultures ................................................................................ -

Multicellular Oxidant Defense in Unicellular Organisms MUCHOU MA and JOHN W
Proc. Natl. Acad. Sci. USA Vol. 89, pp. 7924-7928, September 1992 Microbiology Multicellular oxidant defense in unicellular organisms MUCHOU MA AND JOHN W. EATON* Division of Experimental Pathology, Department of Pathology and Laboratory Medicine, Albany Medical College, A-81, 47 New Scotland Avenue, Albany, NY 12208 Communicated by David W. Talmage, May 8, 1992 ABSTRACT Although catalase is thought to be a major MATERIALS AND METHODS defense against hydrogen peroxide (H202), the catalase activity Reagents. Brain heart infusion broth, Todd-Hewitt broth, within individual Escherichia coil fails to protect against ex- Lennox L agar (LB agar), and Bactoagar were obtained from ogenous H202. Contrary to earlier reports, we find that dilute GIBCO/BRL. The bicinchoninic acid protein microassay suspensions, of wild-type and catalase-deficient E. colt are was from Pierce. All other enzymes and chemicals were identical in their sensitivity to H202, perhaps because even purchased from Sigma. wild-type, catalase-positive E. colU cannot maintain an inter- Bacterial Strains and Culture Conditions. A catalase- nal/externail concentration gradient of this highly diffusible deficient mutant strain of E. coli K-12 [UM1, hereafter, oxidant. However, concentrated suspensions or colonies of cat(-)] and its parent wild-type [CSH7, hereafter cat(+)] (17) catalase-positive E. colt do preferentially survive H202 chal- were provided by P. C. Loewen (University ofManitoba). E. lenge and can even cross-protect adjacent catalase-deficient coli were grown statically in brain heart infusion broth or M9 organisms. Furthermore, high-density catalase-positive-but minimal salts medium supplemented with 10 mM glucose not catalase-negative-E. colt can survive and multiply in the (M9/glucose) (25) at 370C in room air overnight (18-20 hr) to presence of competitive, peroxide-generating streptococci. -

Growth Characteristics of Escherichia Coli and Staphylococcus Aureus Bacteria on Alternative Medium Leaves of Lamtoro (Leucaena Leucocephala)
Journal of Xi'an University of Architecture & Technology ISSN No : 1006-7930 Growth Characteristics of Escherichia coli and Staphylococcus aureus Bacteria on Alternative Medium Leaves of Lamtoro (Leucaena leucocephala) Meidawati Suswandari*, Department of Primary School, Faculty of Teacher Training and Education, Universitas Veteran Bangun Nusantara, Sukoharjo, Indonesia Lamtoro leaf has a high protein content. The protein content is very suitable for bacterial growth. Because of the high cost of bacterial growth media for educational and research institutions, lamtoro leaves can be used as an alternative medium for bacterial growth in general. The purpose of this study was to determine the potential of lamtoro leaf as an alternative medium for bacterial growth in general. This research is descriptive. Alternative mediums of lamtoro leaf were tested for the growth of Escherichia coli and Staphylococcus aureus. Escherichia coli bacteria grow on three alternative medium plates. After final identification, there are Escherichia coli bacteria. Whereas the Staphylococcus aureus bacterium did not grow on seven plates of alternative medium despite being incubated for 48 hours. Lamtoro leaf has less potential as an alternative medium for bacterial growth in general. The lamtoro leaf medium can only be used as a growth medium for gram-negative bacteria. While the growth of gram-positive bacteria there is no growth due to the presence of active substances in lamtoro leaves. Key words: Leaves of Lamtoro, Alternative Media, Escherichia coli, Staphylococcus aureus Introduction Bacteria are single-celled creatures that are very small or microscopic. Hans Christian Gram divides bacteria based on the characteristics of cell walls through the Gram staining system, namely Gram Positive and Gram Negative bacteria (Elferia, et al, 1996; Elliot, 2013; Harvey, 2001; Clausen, Gildberg, and Raa, 1985). -

Introduction to Bacteriology and Bacterial Structure/Function
INTRODUCTION TO BACTERIOLOGY AND BACTERIAL STRUCTURE/FUNCTION LEARNING OBJECTIVES To describe historical landmarks of medical microbiology To describe Koch’s Postulates To describe the characteristic structures and chemical nature of cellular constituents that distinguish eukaryotic and prokaryotic cells To describe chemical, structural, and functional components of the bacterial cytoplasmic and outer membranes, cell wall and surface appendages To name the general structures, and polymers that make up bacterial cell walls To explain the differences between gram negative and gram positive cells To describe the chemical composition, function and serological classification as H antigen of bacterial flagella and how they differ from flagella of eucaryotic cells To describe the chemical composition and function of pili To explain the unique chemical composition of bacterial spores To list medically relevant bacteria that form spores To explain the function of spores in terms of chemical and heat resistance To describe characteristics of different types of membrane transport To describe the exact cellular location and serological classification as O antigen of Lipopolysaccharide (LPS) To explain how the structure of LPS confers antigenic specificity and toxicity To describe the exact cellular location of Lipid A To explain the term endotoxin in terms of its chemical composition and location in bacterial cells INTRODUCTION TO BACTERIOLOGY 1. Two main threads in the history of bacteriology: 1) the natural history of bacteria and 2) the contagious nature of infectious diseases, were united in the latter half of the 19th century. During that period many of the bacteria that cause human disease were identified and characterized. 2. Individual bacteria were first observed microscopically by Antony van Leeuwenhoek at the end of the 17th century. -

“Isolation of Antibiotic Producing Actinomycetes from Soil”
© 2020 JETIR May 2020, Volume 7, Issue 5 www.jetir.org (ISSN-2349-5162) “ISOLATION OF ANTIBIOTIC PRODUCING ACTINOMYCETES FROM SOIL” *Sneha khadse1Priyanka Titirmare2 1) Project assistant at CSIR NEERI Nagpur, 2) Kamla Nehru Mahavidyalaya, Nagpur. ABSTRACT The present study deals with the isolation, production, purification and optimization of antibiotics from soil. The soil samples were collected from various places. Actinomycetes strains were isolated in specific medium using starch casein agar medium. The actinomycetes were screened with regard to potential against gram positive and gram negative bacteria. The purified actinomycetes strains were performed in biochemical tests. Gram staining, sugars fermentation, HS production, urease production, MR-VP test, indole production, citrate 2 utilization. Fermentation was carried out with starch casein broth and identified strain of actinomyces as inoculums. Antimicrobial activity of antibiotics was detected on Mueller Hinton Agar Media against E.coli. The purification of antibiotics was done with the help of activated charcoal. During the experiment a modified fermentation medium was formulated and activity of antibiotic produced in this medium was detected against E.coli. The isolated bacteria such as actinomycetesobtained from the soil. INTRODUCTION Antibiotics are complex chemical secondary metabolites, which are produced by microorganisms & act against other microorganisms. The term antibiotics had its origin from Greek word anti-against+biotikos =”fit for life”. Antibiotics are the best known products of actinomycetes. The best known genus of actinomycetes; Streptomyces (Order Actinomycetales, family Streptomycetaceae are gram-positive, filamentous bacteria that are ubiquitous in soil and produce the majority (>70%) of known antibiotics (Tanaka and Omura, 1990). The isolation & characterization of Actinomycetes were performed in different biochemical methods. -

Laboratory Exercises in Microbiology: Discovering the Unseen World Through Hands-On Investigation
City University of New York (CUNY) CUNY Academic Works Open Educational Resources Queensborough Community College 2016 Laboratory Exercises in Microbiology: Discovering the Unseen World Through Hands-On Investigation Joan Petersen CUNY Queensborough Community College Susan McLaughlin CUNY Queensborough Community College How does access to this work benefit ou?y Let us know! More information about this work at: https://academicworks.cuny.edu/qb_oers/16 Discover additional works at: https://academicworks.cuny.edu This work is made publicly available by the City University of New York (CUNY). Contact: [email protected] Laboratory Exercises in Microbiology: Discovering the Unseen World through Hands-On Investigation By Dr. Susan McLaughlin & Dr. Joan Petersen Queensborough Community College Laboratory Exercises in Microbiology: Discovering the Unseen World through Hands-On Investigation Table of Contents Preface………………………………………………………………………………………i Acknowledgments…………………………………………………………………………..ii Microbiology Lab Safety Instructions…………………………………………………...... iii Lab 1. Introduction to Microscopy and Diversity of Cell Types……………………......... 1 Lab 2. Introduction to Aseptic Techniques and Growth Media………………………...... 19 Lab 3. Preparation of Bacterial Smears and Introduction to Staining…………………...... 37 Lab 4. Acid fast and Endospore Staining……………………………………………......... 49 Lab 5. Metabolic Activities of Bacteria…………………………………………….…....... 59 Lab 6. Dichotomous Keys……………………………………………………………......... 77 Lab 7. The Effect of Physical Factors on Microbial Growth……………………………... 85 Lab 8. Chemical Control of Microbial Growth—Disinfectants and Antibiotics…………. 99 Lab 9. The Microbiology of Milk and Food………………………………………………. 111 Lab 10. The Eukaryotes………………………………………………………………........ 123 Lab 11. Clinical Microbiology I; Anaerobic pathogens; Vectors of Infectious Disease….. 141 Lab 12. Clinical Microbiology II—Immunology and the Biolog System………………… 153 Lab 13. Putting it all Together: Case Studies in Microbiology…………………………… 163 Appendix I. -

Biochemical Media Casitone Broth-White Cap with Black Circle. Loop Transfer
Biochemical Media Casitone Broth-white cap with black circle. Loop transfer. (Use care tops may be very loose) (Do not put caps on the wrong tubes during the inoculation procedures). Can the organism split tryptophan into indole, pyruvic acid and ammonia? Test for the presence of indole (unique to this process) by adding 5 drops of Kovacs Test Reagent. If indole is present, a ruby red color will form at the top of the test tube. The media name for casitone comes from the word casein. Casein is a milk protein rich in the amino acid tryptophan. Result sheet recorded with a “pos” or “neg” Phenol Red Carbohydrate Fermentation Broths (Phenol Reds) Red capped broth with durham tube-Phenol Red with Lactose Clear capped broth with durham tube-Phenol Red with Glucose Yellow capped broth with durham tube-Phenol Red with Sucrose Blue capped broth with durham tube-Phenol Red with Maltose Green capped broth with durham tube-Phenol Red with Mannitol All tubes are inoculated with loop transfers. Can the organism ferment the carbohydrates listed above? Each comes with a separate sugar, a durham tube for gas collection and the acid-base indicator called phenolphthalein. Fermentation will produce acidity and sometimes gas. Gas alone is not produced by the fermentation process. Phenolphthalein will turn yellow in the presence of acid, red in an alkaline environment. Gas production can be observed by the presence of air bubbles captured in the durham tube. For each result both an acid and gas response should be indicated. For a large amount of acid (yellow) use “A”. -

Applications of Microscopy in Bacteriology
Microscopy Research, 2016, 4, 1-9 Published Online January 2016 in SciRes. http://www.scirp.org/journal/mr http://dx.doi.org/10.4236/mr.2016.41001 Applications of Microscopy in Bacteriology Mini Mishra1, Pratima Chauhan2* 1Centre of Environmental Studies, Department of Botany, University of Allahabad, Allahabad, India 2Department of Physics, University of Allahabad, Allahabad, India Received 28 September 2015; accepted 2 January 2016; published 5 January 2016 Copyright © 2016 by authors and Scientific Research Publishing Inc. This work is licensed under the Creative Commons Attribution International License (CC BY). http://creativecommons.org/licenses/by/4.0/ Abstract Bacteria are smallest primitive, simple, unicellular, prokaryotic and microscopic organisms. But these organisms cannot be studied with naked eyes because of their minute structure. Therefore in search for the information about the structure and composition of bacterial cells, cell biologist used light microscopes with a numerical aperture of 1.4 and using wavelength of 0.4 µm separa- tion. But there are still certain cellular structures that cannot be seen through naked eyes, and for them electron microscope is used. There are certain improved types of light microscope which can be incorporated to improve their resolving power. Hence microscopy is playing a crucial role in the field of bacteriology. Keywords AFM, SEM, TEM, Microscopy, Bacteriology 1. Introduction To get acquainted with the world of bacteria like small organisms, very effective and advanced technique is re- quired. The size of bacteria ranges between 0.5 - 5.0 micrometer in length; the smallest of them are members of mycoplasma which measures 0.3 micrometers [1]. -

Medical Bacteriology
LECTURE NOTES Degree and Diploma Programs For Environmental Health Students Medical Bacteriology Abilo Tadesse, Meseret Alem University of Gondar In collaboration with the Ethiopia Public Health Training Initiative, The Carter Center, the Ethiopia Ministry of Health, and the Ethiopia Ministry of Education September 2006 Funded under USAID Cooperative Agreement No. 663-A-00-00-0358-00. Produced in collaboration with the Ethiopia Public Health Training Initiative, The Carter Center, the Ethiopia Ministry of Health, and the Ethiopia Ministry of Education. Important Guidelines for Printing and Photocopying Limited permission is granted free of charge to print or photocopy all pages of this publication for educational, not-for-profit use by health care workers, students or faculty. All copies must retain all author credits and copyright notices included in the original document. Under no circumstances is it permissible to sell or distribute on a commercial basis, or to claim authorship of, copies of material reproduced from this publication. ©2006 by Abilo Tadesse, Meseret Alem All rights reserved. Except as expressly provided above, no part of this publication may be reproduced or transmitted in any form or by any means, electronic or mechanical, including photocopying, recording, or by any information storage and retrieval system, without written permission of the author or authors. This material is intended for educational use only by practicing health care workers or students and faculty in a health care field. PREFACE Text book on Medical Bacteriology for Medical Laboratory Technology students are not available as need, so this lecture note will alleviate the acute shortage of text books and reference materials on medical bacteriology. -

History of the Department of Microbiology 1868 – 2009
June 2015 HISTORY OF THE DEPARTMENT OF MICROBIOLOGY 1868 – 2009 University of Illinois at Urbana-Champaign 1 A HISTORY OF THE DEPARTMENT OF MICROBIOLOGY 1868 – 2009 This 141 year history of the Department of Microbiology includes an article (Chapter 1), written and published in 1959 by the Department, which covers the period 1868 to 1959. I joined the Department in 1953, and my recounting of the Department’s history includes personal observations as well as anecdotes told to me by H. O. Halvorson and others. Later I realized what a unique experience it had been to join a first-class department, and I resolved to play a role in maintaining its research stature. Ralph Wolfe 2 Department of Microbiology History of the Headship: 1950 – 1959 Halvor Halvorson 1960 – 1963 Kim Atwood 1963 – 1972 Leon Campbell 1972 – 1982 Ralph DeMoss 1982 – 1987 Samuel Kaplan 1987 – 1990 Jordan Konisky 1990 – 1991 Ralph Wolfe (Acting Head) 1991 – 1997 Charles Miller 1997 – 2002 John Cronan 2003 – 2004 Jeffrey Gardner (Acting Head) 2005 – Present John Cronan 3 Organization of the History of the Department In Chapters 2 to 6 the data are divided into Academic Decades, each containing the following sections: Section I, an overview of the decade; Section II, some events for each year of the decade; Section III, a summary of the research interests, honors received, publications, and invited off-campus lectures or seminars for each faculty member. These data have been obtained from the annual reports of the faculty submitted to the departmental secretary. 4 CHAPTER 1 1868 – 1959 During this time period the name of the Department was Department of Bacteriology (Anecdotes by Ralph Wolfe) A SHORT HISTORY OF THE DEPARTMENT OF BACTERIOLOGY H. -
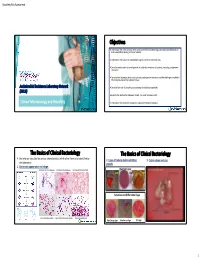
Objectives the Basics of Clinical Bacteriology the Basics of Clinical
Biosafety Risk Assessment Objectives Understand the most common tests used by the clinical bacteriology laboratory for identification and susceptibility testing of clinical isolates. Understand the classes of antimicrobial agents and their potential uses. Describe mechanisms for development of antibiotic resistance in bacteria, including carbapenem resistance. Describe the laboratory tests used to detect carbapenem resistance and the challenges involved in the interpretation of the laboratory data. Antimicrobial Resistance Laboratory Network Describe the role of biosafety in protecting the healthcare provider. (ARLN) Explain the relationship between hazard, risk, and risk assessment. Clinical Microbiology and Biosafety Understand the Antibiotic Resistance Laboratory Network initiative. The Basics of Clinical Bacteriology The Basics of Clinical Bacteriology Bacteria are classified by various characteristics which allow them to be identified by 2. Types of culture media exhibiting 3. Colony shape and size: the laboratory: growth: 1. Gram stain appearance and shape: Gram Positive Cocci in chains (purple) Gram Positive Diplococci (purple) Gram Negative Diplococci (red/pink) Nutrient Agar BAP BAP BAP Selective and Differential Agar Gram Negative Rods(red/pink) Gram Positive Cocci in Clusters (purple) Gram Positive Rods (purple) MacConkey Agar MacConkey Agar XLD Agar 1 Biosafety Risk Assessment The Basics of Clinical Bacteriology The Basics of Clinical Bacteriology 4. Atmospheric requirements for bacterial growth: 5. Organism Identification: Spot tests – rapid biochemical tests which can be used to rule in/out various groups of organisms Catalase: Ability to breakdown H2O2 Oxidase: Presence of cytochrome oxidase CO2 – Neisseria spp., Haemophilus spp., Streptococcus pneumonia 2 Microaerophilic ( reduced O ) – Campylobacter spp. (+) Staphylococcus spp. (=) Streptococcus spp. Pseudomonas spp. E. coli (Enterobacteriaceae) Anaerobic (lack of O2) – Clostridium difficile The Basics of Clinical Bacteriology The Basics of Clinical Bacteriology 5. -

Canada 57-2 Fisheries Peches L+I and Oceans Et Oceans STANDARD PROCEDURES PROCEDURES POUR for BACTERIOLOGICAL ANALYSES ANALYSIS BACTERIOLOGIQUES
/0.58:29 Fisheries Peches l+I and Oceans et Oceans Inspection Services de Services )'inspection Standard Procedures pour Procedures for analyses Bacteriological bacteriologiques Analysis ( /v/fJ,rJ Ul.LS IX 54.3 Canada 57-2 Fisheries Peches l+I and Oceans et Oceans STANDARD PROCEDURES PROCEDURES POUR FOR BACTERIOLOGICAL ANALYSES ANALYSIS BACTERIOLOGIQUES Record of Amendments amend. no chapter date of amend. inserted by insertion date 1 5 +Qn1"' E 31 - ns- X'S C? c l~ Le.Lle.J/ gs - Dtc C; ··7 ~ />f» c ~ e:. ~.J J-~ \ s .. o sfft8 /vf:-l~ fb. ~-o 1~1r . Government Gouvernement of Canada du Canada IDate ••• Canadian Food Agence canadienne 1 15£05£98 Inspection Agency d'inspection des aliments STANDARD PROCEDURES PROCEDURES POUR FOR BACTERIOLOGICAL ANALYSES ANALYSIS BACTERIOLOGIQUES Bulletin TO: All Holders of the Standard Procedures for Bacteriological Analysis Manual SUBJECT: MODIFICATIONS TO THE PROCEDURES Attached are modifications to various Chapters and Appendices of the Bacteriological Analysis manual. The changes have been incorporated in this bulletin format in order to provide the updates to manual holders as quickly as possible and also due to the uncertainty as to whether the manual will continue to be used as a bacteriological procedures reference by the Agency. The changes to the respective sections have been incorporated on separate pages so the pertinent page(s) may be inserted with the relevant chapter/appendix. If required, these modifications will be incorporated in the manual at a later date. Cam Prince Director Fish,