Isotopic Assessment of Long Term Groundwater Exploitation
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Land Tenure Reforms and Social Transformation in Botswana: Implications for Urbanization
Land Tenure Reforms and Social Transformation in Botswana: Implications for Urbanization. Item Type text; Electronic Dissertation Authors Ijagbemi, Bayo, 1963- Publisher The University of Arizona. Rights Copyright © is held by the author. Digital access to this material is made possible by the University Libraries, University of Arizona. Further transmission, reproduction or presentation (such as public display or performance) of protected items is prohibited except with permission of the author. Download date 06/10/2021 17:13:55 Link to Item http://hdl.handle.net/10150/196133 LAND TENURE REFORMS AND SOCIAL TRANSFORMATION IN BOTSWANA: IMPLICATIONS FOR URBANIZATION by Bayo Ijagbemi ____________________ Copyright © Bayo Ijagbemi 2006 A Dissertation Submitted to the Faculty of the DEPARTMENT OF ANTHROPOLOGY In Partial Fulfillment of the Requirements For the Degree of DOCTOR OF PHILOSOPHY In the Graduate College THE UNIVERSITY OF ARIZONA 2006 2 THE UNIVERSITY OF ARIZONA GRADUATE COLLEGE As members of the Dissertation Committee, we certify that we have read the dissertation prepared by Bayo Ijagbemi entitled “Land Reforms and Social Transformation in Botswana: Implications for Urbanization” and recommend that it be accepted as fulfilling the dissertation requirement for the Degree of Doctor of Philosophy _______________________________________________________________________ Date: 10 November 2006 Dr Thomas Park _______________________________________________________________________ Date: 10 November 2006 Dr Stephen Lansing _______________________________________________________________________ Date: 10 November 2006 Dr David Killick _______________________________________________________________________ Date: 10 November 2006 Dr Mamadou Baro Final approval and acceptance of this dissertation is contingent upon the candidate’s submission of the final copies of the dissertation to the Graduate College. I hereby certify that I have read this dissertation prepared under my direction and recommend that it be accepted as fulfilling the dissertation requirement. -

Botswana Semiology Research Centre Project Seismic Stations In
BOTSWANA SEISMOLOGICAL NETWORK ( BSN) STATIONS 19°0'0"E 20°0'0"E 21°0'0"E 22°0'0"E 23°0'0"E 24°0'0"E 25°0'0"E 26°0'0"E 27°0'0"E 28°0'0"E 29°0'0"E 30°0'0"E 1 S 7 " ° 0 0 ' ' 0 0 ° " 7 S 1 KSANE Kasane ! !Kazungula Kasane Forest ReserveLeshomo 1 S Ngoma Bridge ! 8 " ! ° 0 0 ' # !Mabele * . MasuzweSatau ! ! ' 0 ! ! Litaba 0 ° Liamb!ezi Xamshiko Musukub!ili Ivuvwe " 8 ! ! ! !Seriba Kasane Forest Reserve Extension S 1 !Shishikola Siabisso ! ! Ka!taba Safari Camp ! Kachikau ! ! ! ! ! ! Chobe Forest Reserve ! !! ! Karee ! ! ! ! ! Safari Camp Dibejam!a ! ! !! ! ! ! ! X!!AUD! M Kazuma Forest Reserve ! ShongoshongoDugamchaRwelyeHau!xa Marunga Xhauga Safari Camp ! !SLIND Chobe National Park ! Kudixama Diniva Xumoxu Xanekwa Savute ! Mah!orameno! ! ! ! Safari Camp ! Maikaelelo Foreset Reserve Do!betsha ! ! Dibebe Tjiponga Ncamaser!e Hamandozi ! Quecha ! Duma BTLPN ! #Kwiima XanekobaSepupa Khw!a CHOBE DISTRICT *! !! ! Manga !! Mampi ! ! ! Kangara # ! * Gunitsuga!Njova Wazemi ! ! G!unitsuga ! Wazemi !Seronga! !Kaborothoa ! 1 S Sibuyu Forest Reserve 9 " Njou # ° 0 * ! 0 ' !Nxaunxau Esha 12 ' 0 Zara ! ! 0 ° ! ! ! " 9 ! S 1 ! Mababe Quru!be ! ! Esha 1GMARE Xorotsaa ! Gumare ! ! Thale CheracherahaQNGWA ! ! GcangwaKaruwe Danega ! ! Gqose ! DobeQabi *# ! ! ! ! Bate !Mahito Qubi !Mahopa ! Nokaneng # ! Mochabana Shukumukwa * ! ! Nxabe NGAMILAND DISTRICT Sorob!e ! XurueeHabu Sakapane Nxai National Nark !! ! Sepako Caecae 2 ! ! S 0 " Konde Ncwima ° 0 ! MAUN 0 ' ! ! ' 0 Ntabi Tshokatshaa ! 0 ° ! " 0 PHDHD Maposa Mmanxotai S Kaore ! ! Maitengwe 2 ! Tsau Segoro -

E-Government and Democracy in Botswana: Observational and Experimental Evidence on the Effects of E-Government Usage on Political Attitudes
A Service of Leibniz-Informationszentrum econstor Wirtschaft Leibniz Information Centre Make Your Publications Visible. zbw for Economics Bante, Jana et al. Working Paper E-government and democracy in Botswana: Observational and experimental evidence on the effects of e-government usage on political attitudes Discussion Paper, No. 16/2021 Provided in Cooperation with: German Development Institute / Deutsches Institut für Entwicklungspolitik (DIE), Bonn Suggested Citation: Bante, Jana et al. (2021) : E-government and democracy in Botswana: Observational and experimental evidence on the effects of e-government usage on political attitudes, Discussion Paper, No. 16/2021, ISBN 978-3-96021-153-2, Deutsches Institut für Entwicklungspolitik (DIE), Bonn, http://dx.doi.org/10.23661/dp16.2021 This Version is available at: http://hdl.handle.net/10419/234177 Standard-Nutzungsbedingungen: Terms of use: Die Dokumente auf EconStor dürfen zu eigenen wissenschaftlichen Documents in EconStor may be saved and copied for your Zwecken und zum Privatgebrauch gespeichert und kopiert werden. personal and scholarly purposes. Sie dürfen die Dokumente nicht für öffentliche oder kommerzielle You are not to copy documents for public or commercial Zwecke vervielfältigen, öffentlich ausstellen, öffentlich zugänglich purposes, to exhibit the documents publicly, to make them machen, vertreiben oder anderweitig nutzen. publicly available on the internet, or to distribute or otherwise use the documents in public. Sofern die Verfasser die Dokumente unter Open-Content-Lizenzen (insbesondere CC-Lizenzen) zur Verfügung gestellt haben sollten, If the documents have been made available under an Open gelten abweichend von diesen Nutzungsbedingungen die in der dort Content Licence (especially Creative Commons Licences), you genannten Lizenz gewährten Nutzungsrechte. -

Hydro-Geochemical Processes in the Complexe Terminal
Journal of African Earth Sciences 100 (2014) 81–95 Contents lists available at ScienceDirect Journal of African Earth Sciences journal homepage: www.elsevier.com/locate/jafrearsci Hydro-geochemical processes in the Complexe Terminal aquifer of southern Tunisia: An integrated investigation based on geochemical and multivariate statistical methods ⇑ Friha Hadj Ammar a,c,1, , Najiba Chkir a,b,2, Kamel Zouari a,2, Bruno Hamelin c,3, Pierre Deschamps c,3, Aissa Aigoun d,4 a Laboratory of Radio-Analysis and Environment, National School of Engineering of Sfax, Route de Soukra, BP 1173, 3038 Sfax, Tunisia b Laboratory of Radio-Analysis and Environment, Faculty of Letters and Humanities, Geography Department, Route El Matar, BP 1168, 3029 Sfax, Tunisia c CEREGE, UMR Aix-Marseille Université, CNRS, IRD, Collège de France, Europôle de l’Arbois, BP 80, 13545 Aix en Provence cedex 04, France d Commissariat Régional de développement Agricole Kébili, Rue Salah Ben Youssef 4200 Kébili, Tunisia article info abstract Article history: Hydrochemical data from a total of 104 groundwater samples were used to investigate the main factors Received 30 November 2013 and mechanisms that control the chemistry of groundwaters in the Complexe Terminal (CT) aquifer of Received in revised form 20 June 2014 Chott region in southern Tunisia. Multivariate statistical techniques combining Hierarchical Cluster Anal- Accepted 21 June 2014 ysis (HCA) and Principal Component Analysis (PCA) were applied to the dataset of 12 physicochemical Available online 28 June 2014 + + 2+ 2+ À À À 2À parameters (i.e. pH, T°, depth, Na ,K,Ca ,Mg , HCO3 ,NO3 ,Cl ,SO4 , and TDS). -

Establishment of Subordinate Land Boards (Amendment) Order
CHAPTER 32:02 - TRIBAL LAND: SUBSIDIARY LEGISLATION INDEX TO SUBSIDIARY LEGISLATION Establishment of Subordinate Land Boards (Amendment) Order Establishment of Subordinate Land Boards Order Tribal Land (Establishment of Land Tribunals) Order Tribal Land (Subordinate Land Boards) Regulations Tribal Land Regulations ESTABLISHMENT OF SUBORDINATE LAND BOARDS ORDER (under section 19) (15th June, 1973) ARRANGEMENT OF PARAGRAPHS PARAGRAPH 1. Citation 2. Establishment 3. Area of jurisdiction 4. Functions Schedule S.I. 47, 1973, S.I. 3, 1979, S.I. 125, 1979, S.I. 132, 1980, S.I. 78, 1981, S.I. 81, 1981, S.I. 110, 1981, S.I. 68, 1982, S.I. 5, 1984, S.I. 92, 1984, S.I. 36, 1986, S.I. 55,1987, S.I. 97, 1989, S.I. 45, 1992, S.I. 66, 1994, S.I. 53, 2002. 1. Citation Copyright Government of Botswana This Order may be cited as the Establishment of Subordinate Land Boards Order. 2. Establishment The subordinate land boards referred to in the second column of the Schedule hereto are established as the subordinate land boards within the district named in the first column of the said Schedule. 3. Area of jurisdiction The area of jurisdiction in respect of which each subordinate Land Board will perform its functions shall be the area or villages stated in relation to each subordinate land board in the third column of the Schedule. 4. Functions (1) The functions under customary law which vest in the subordinate land authority which are transferred to the subordinate land board shall include the hearing, grant or refusal of applications to use land for— ( a) building residences or extensions thereto; ( b) ploughing to a maximum extent of land determined by the tribal land board; ( c) grazing cattle or other stock; ( d) communal uses in the village. -

Fonctionnement Et Salinité De La Nappe De L'oasis De Fatnassa : Arguments Géochimiques
M. Kuper, A. Zaïri, (éditeurs scientifiques) 2008. Economies d’eau en systèmes irrigués au Maghreb. Actes du troisième atelier régional du projet Sirma, Nabeul, Tunisie, 4-7 juin 2007. Cirad, Montpellier, France, colloques-cédérom. Fonctionnement et salinité de la nappe de l'oasis de Fatnassa : arguments géochimiques MARLET S.1,2, BOUKSILA F.2, MEKKI I.2,, BENAISSA I.2 1 Cirad, 34398 Montpellier Cedex 5, France, [email protected] 2 INRGREF, Institut national de recherche en génie rural, eau et forêt, BP 10, 2080 Ariana, Tunis, Tunisie Résumé — Les sols et les eaux de la nappe du périmètre oasien de Fatnassa Nord (114 hectares) sont menacés par un risque de salinisation en raison d’une salinité élevée des eaux d’irrigation et de la position superficielle de la nappe. Un réseau de 27 piézomètres a été installé depuis 2001 pour le suivi de la profondeur, de la salinité et de la composition chimique de la nappe. La composition chimique de la nappe apparaît principalement liée à la concentration des eaux d’irrigation et à la précipitation de gypse et de calcite, et secondairement aux variations de la pression partielle en CO2 influençant la teneur en carbonate et le pH. Ces processus ont été simulés avec le modèle IRRICHEM. Les ions chlorure n’interviennent pas dans les mécanismes géochimiques et ont été retenus comme indicateur du facteur de concentration des eaux de la nappe par rapport aux eaux d’irrigation. Le facteur de concentration des eaux de la nappe varie d’un facteur 1,7 à 7,7 par rapport aux eaux d’irrigation (médiane de 4,41) tandis que le facteur de concentration des eaux de drainage est de 3,41. -
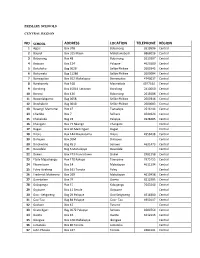
Public Primary Schools
PRIMARY SCHOOLS CENTRAL REGION NO SCHOOL ADDRESS LOCATION TELE PHONE REGION 1 Agosi Box 378 Bobonong 2619596 Central 2 Baipidi Box 315 Maun Makalamabedi 6868016 Central 3 Bobonong Box 48 Bobonong 2619207 Central 4 Boipuso Box 124 Palapye 4620280 Central 5 Boitshoko Bag 002B Selibe Phikwe 2600345 Central 6 Boitumelo Bag 11286 Selibe Phikwe 2600004 Central 7 Bonwapitse Box 912 Mahalapye Bonwapitse 4740037 Central 8 Borakanelo Box 168 Maunatlala 4917344 Central 9 Borolong Box 10014 Tatitown Borolong 2410060 Central 10 Borotsi Box 136 Bobonong 2619208 Central 11 Boswelakgomo Bag 0058 Selibe Phikwe 2600346 Central 12 Botshabelo Bag 001B Selibe Phikwe 2600003 Central 13 Busang I Memorial Box 47 Tsetsebye 2616144 Central 14 Chadibe Box 7 Sefhare 4640224 Central 15 Chakaloba Bag 23 Palapye 4928405 Central 16 Changate Box 77 Nkange Changate Central 17 Dagwi Box 30 Maitengwe Dagwi Central 18 Diloro Box 144 Maokatumo Diloro 4958438 Central 19 Dimajwe Box 30M Dimajwe Central 20 Dinokwane Bag RS 3 Serowe 4631473 Central 21 Dovedale Bag 5 Mahalapye Dovedale Central 22 Dukwi Box 473 Francistown Dukwi 2981258 Central 23 Etsile Majashango Box 170 Rakops Tsienyane 2975155 Central 24 Flowertown Box 14 Mahalapye 4611234 Central 25 Foley Itireleng Box 161 Tonota Foley Central 26 Frederick Maherero Box 269 Mahalapye 4610438 Central 27 Gasebalwe Box 79 Gweta 6212385 Central 28 Gobojango Box 15 Kobojango 2645346 Central 29 Gojwane Box 11 Serule Gojwane Central 30 Goo - Sekgweng Bag 29 Palapye Goo-Sekgweng 4918380 Central 31 Goo-Tau Bag 84 Palapye Goo - Tau 4950117 -

Daily Hansard 17 March 2021
DAILY YOUR VOICE IN PARLIAMENT THE SECONDSECOND MEETING MEETING OF THEOF SECONDTHE FIFTH SESSION SESSION OF THEOF THEELEVEN TWELFTHTH PARLIAMENT PARLIAMENT WEDNESDAY 17 MARCH 2021 MIXEDMIXED VERSION VERSION HANSARDHANSARD NO. 193201 DISCLAIMER Unocial Hansard This transcript of Parliamentary proceedings is an unocial version of the Hansard and may contain inaccuracies. It is hereby published for general purposes only. The nal edited version of the Hansard will be published when available and can be obtained from the Assistant Clerk (Editorial). THE NATIONAL ASSEMBLY SPEAKER The Hon. Phandu T. C. Skelemani PH, MP. DEPUTY SPEAKER The Hon. Mabuse M. Pule, MP. (Mochudi East) Clerk of the National Assembly - Ms B. N. Dithapo Deputy Clerk of the National Assembly - Mr L. T. Gaolaolwe Learned Parliamentary Counsel - Ms M. Mokgosi Assistant Clerk (E) - Mr R. Josiah CABINET His Excellency Dr M. E. K. Masisi, MP. - President His Honour S. Tsogwane, MP. (Boteti West) - Vice President Minister for Presidential Affairs, Governance and Public Hon. K. N. S. Morwaeng, MP. (Molepolole South) - Administration Hon. K. T. Mmusi, MP. (Gabane-Mmankgodi) - Minister of Defence, Justice and Security Hon. Dr L. Kwape, MP. (Kanye South) - Minister of International Affairs and Cooperation Hon. E. M. Molale, MP. (Goodhope-Mabule ) - Minister of Local Government and Rural Development Hon. K. S. Gare, MP. (Moshupa-Manyana) - Minister of Agricultural Development and Food Security Minister of Environment, Natural Resources Conservation Hon. P. K. Kereng, MP. (Specially Elected) - and Tourism Hon. Dr E. G. Dikoloti MP. (Mmathethe-Molapowabojang) - Minister of Health and Wellness Hon. T.M. Segokgo, MP. (Tlokweng) - Minister of Transport and Communications Hon. -

Amendmt-CNEA APIA
Introduction La culture du palmier dattier occupe une place de choix dans l’agriculture Tunisienne puis qu’en plus de son adaptation au milieu désertique, elle a permis d’optimiser l’exploitation des ressources hydrauliques du Sud et a participé d’une manière significative au produit du secteur agricole et à la création de sources de revenus substantiels des habitants dans les oasis.La présence de sources d’eau naturelles a permis de développer le système oasien spécifique aux régions de montagne telle Tameghza et aux régions de plaines telle que Tozeur et Kebili. C’est dans ce milieu présentant des potentialités importantes (eau, sol) qui s’est développée les oasis traditionnelles caractérisées par la densité élevée des plantations de palmiers, l’existence de système de production associant aux palmiers l’arboriculture fruitière et les cultures d’hiver et d’été et permettant d’ancrer une dynamique économique dans ces régions. Les populations résidentes qui y sont rattachées ont pu, au fil des années, améliorer leurs revenus agricoles, tirer profit des opportunités du marché d’exportation des dattes et de l’amélioration des conditions de commercialisation de ce produit et sont devenues de plus en plus enthousiastes à étendre les superficies exploitées quitte même à faire des extensions illicites et à procéder à différentes techniques de préservation de leur espace. De par le revenu dégagé de l’exploitation des palmiers, il y’a la main d’œuvre agricole et la dynamique économique qui sont créées dans chaque région d’oasis. Mais au fur et à mesure de l’avancement de l’age des plantations de palmier dattier, de l’exploitation parfois anarchique et souvent démesurée des facteurs sol et eau et du manque d’apport de matières organiques, il s’est apparu un appauvrissement des sols, qui s’est vite traduit en baisse des rendements et en recul du revenu de l’agriculteur. -

Assessment of Aquifer Mixing and Salinity Intrusion in the North-Western Sahara Aquifer System: a Hydrogeochemical Analysis- Algeria, Tunisia
Assessment of Aquifer Mixing and Salinity Intrusion in the North-Western Sahara Aquifer System: a Hydrogeochemical Analysis- Algeria, Tunisia By David F. Meyer B.S., Kansas State University, 2011 Submitted to the graduate degree program in the Department of Geology and Graduate Faculty of the University of Kansas in partial fulfillment of the requirements for the Degree of Master of Science Advisory Committee ________________________________ Randy Stotler (Chair) ________________________________ G. L. Macpherson (Co-Chair) ________________________________ J.F. Devlin ________________________________ W.C. Johnson Date Defended: October 27, 2016 The Thesis committee for David Meyer certifies that this is the approved version of the following thesis: Assessment of Aquifer Mixing and Salinity Intrusion in the North-Western Sahara Aquifer System: a Hydrogeochemical Analysis- Algeria, Tunisia ________________________________ Randy Stotler (Chair) ________________________________ G. L. Macpherson (Co-Chair) Date Approved: December 7, 2016 ii Abstract The North-Western Sahara Aquifer System is a complex multilayer leaky aquifer system providing water to Algeria, Tunisia, and Libya. Changing the hydrologic equilibrium through pumping can cause previously isolated saline water to mix with the fresh water in the pumped aquifers, resulting in increased salinity over time and, creating the potential for water-related conflict. The objective of this study is to identify areas where salinity intrusion is occurring now and could worsen in the future. To accomplish this, fourteen existing datasets were analyzed, yielding new insights into regional and local occurrences of salinity intrusion. Major ion chemistry and ratios of Br/Cl indicate that the source of salinity in the saline aquifers of the system, which intrude into the freshwater, is the dissolution of evaporates in the aquifer matrix. -

World Bank Document
Document of The World Bank FOR OFFICIAL USE ONLY Public Disclosure Authorized Report No. 6245 Public Disclosure Authorized PROJECT PERFORMANCE AUDIT REPORT BOTSWANA SECOND LIVESTOCK DEVELOPMENT PROJECT (LOAN 1497-BT) Public Disclosure Authorized June 13, 1986 Public Disclosure Authorized Operations Evaluation Department This document has a restricted distribution and may be used by recipients only in the performance of their official duties. Its contents may not otherwise be disclosed without World Bank authorization. ABBREVIATIONS AMA - Agricultural K.,Lagement Associations APRU - Animal Production Research Unit BLDC - Botswana Livestock Development Corporation BMC - Botswana Meat Corporation CGC - Communal Grazing Cell CGU - Communal Grazing Unit DAH - Department of Animal Health DWA - Department of Water Affairs EDF - European Development Fund ERR - Economic Rate of Return FA0 - Food and Agriculture Organization FMD - Foot and Mouth Disease GOB - Government of Botswana ILCA - International Livestock Center for Africa LP1 - (First) Livestock Development Proje.:t (Credit 325-BT) LP2 - Second Livestock Development Project (Loan 1497-BT) LPCU - Livestock Project Coordinating Unit - LPMU - Livestock Project Management Unit LUPAGS - Land Use Planning Advisory Groups MOA - Ministry of Agriculture M&E - Monitoring and Evaluation MFDP - Ministry of Finance and Development Planning NDB - National Development Bank NLMLP - National Land Management and Livestock Project NTRP - National Trek Route Policy OED - Operations Evaluation Department PC -

De L'identification Des Contraintes Environnementales À
De l’identification des contraintes environnementales à l’évaluation des performances agronomiques dans un système irrigué collectif. Cas de l’oasis de Fatnassa (Nefzaoua, sud tunisien). Wafa Ghazouani To cite this version: Wafa Ghazouani. De l’identification des contraintes environnementales à l’évaluation des performances agronomiques dans un système irrigué collectif. Cas de l’oasis de Fatnassa (Nefzaoua, sud tunisien).. domain_other. AgroParisTech, 2009. Français. tel-00473373v1 HAL Id: tel-00473373 https://tel.archives-ouvertes.fr/tel-00473373v1 Submitted on 15 Apr 2010 (v1), last revised 19 Apr 2010 (v2) HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. N° /_/_/_/_/_/_/_/_/_/_/ THÈSE Présentée pour obtenir le grade de Docteur de l’Institut des Sciences et Industries du Vivant et de l’Environnement (AgroParisTech) Spécialité : Sciences de l’eau Par Wafa GHAZOUANI De l’identification des contraintes environnementales à l’évaluation des performances agronomiques dans un système irrigué collectif. Cas de l’oasis de Fatnassa (Nefzaoua, sud tunisien). Soutenue publiquement le 16 décembre 2009 A l’Ecole Nationale du génie Rural, des Eaux et des Forêts, Montpellier, France Devant le jury composé de : M.