Extraction and Potential of Cinnamon Essential Oil Towards Repellency and Insecticidal Activity
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Juniperus Communis L.) Essential Oil
Antioxidants 2014, 3, 81-98; doi:10.3390/antiox3010081 OPEN ACCESS antioxidants ISSN 2076-3921 www.mdpi.com/journal/antioxidants Article Chemical Composition and Antioxidant Properties of Juniper Berry (Juniperus communis L.) Essential Oil. Action of the Essential Oil on the Antioxidant Protection of Saccharomyces cerevisiae Model Organism Martina Höferl 1,*, Ivanka Stoilova 2, Erich Schmidt 1, Jürgen Wanner 3, Leopold Jirovetz 1, Dora Trifonova 2, Lutsian Krastev 4 and Albert Krastanov 2 1 Department of Pharmaceutical Chemistry, Division of Clinical Pharmacy and Diagnostics, University of Vienna, Vienna 1090, Austria; E-Mails: [email protected] (E.S.); [email protected] (L.J.) 2 Department Biotechnology, University of Food Technologies, Plovdiv 4002, Bulgaria; E-Mails: [email protected] (I.S.); [email protected] (D.T.); [email protected] (A.K.) 3 Kurt Kitzing Co., Wallerstein 86757, Germany; E-Mail: [email protected] 4 University Laboratory for Food Analyses, University of Food Technologies, Plovdiv 4002, Bulgaria; E-Mail: [email protected] * Author to whom correspondence should be addressed; E-Mail: [email protected]; Tel.: +43-1-4277-55555; Fax: +43-1-4277-855555. Received: 11 December 2013; in revised form: 26 January 2014 / Accepted: 28 January 2014 / Published: 24 February 2014 Abstract: The essential oil of juniper berries (Juniperus communis L., Cupressaceae) is traditionally used for medicinal and flavoring purposes. As elucidated by gas chromatography/flame ionization detector (GC/FID) and gas chromatography/mass spectrometry (GC/MS methods), the juniper berry oil from Bulgaria is largely comprised of monoterpene hydrocarbons such as α-pinene (51.4%), myrcene (8.3%), sabinene (5.8%), limonene (5.1%) and β-pinene (5.0%). -

Ceylon Cinnamon • Pure Cinnamon • Mexican Cinnamon • Sri Lanka Cinnamon • Canela (Spanish for Cinnamon)
Cinnamon The 4th most valuable spice in the world http://www.trueceylonspices.com/ceylon-cinnamon/ www.truecylonspices.com 1. About Cinnamon is a first traded and most popular spice from the ancient time. It extracts from the bark of the cinnamon tree have also been used traditionally as medicine throughout the world. www.truecylonspices.com 2. Products of Cinnamon • Cinnamon Quills (Full tubes) • Cinnamon Quillings (broken tubes) • Cinnamon Featherings • Cinnamon Chips • Ground Cinnamon (Cinnamon powder) • Cinnamon Leaf Oil • Cinnamon Bark Oil www.truecylonspices.com 3. Varieties 1. Cassia Cinnamon 1. Cinnamomum loureiroi 2. Cinnamomum aromaticum 3. Cinnamomum burmannii 2. True Cinnamon 1. Cinnamomum verum www.truecylonspices.com 3.1.1 Cinnamomum loureiroi • Other names: • Saigon Cinnamon • Vietnamese cinnamon • Vietnamese cassia • Origin: • Vietnam • Pros: • Strong spicy cinnamon taste • high levels of oil content Image credit: Wikipedia • Cons: • High Coumarin Levels www.truecylonspices.com 3.1.2 Cinnamomum aromaticum • Other names: • Cinnamomum cassia (old Latin name) • Cassia • Chinese cinnamon • Chinese cassia • Tung Hing • Origin: • China • Pros: • Cheap Image credit: Wikipedia • Cons: • High Coumarin Levels www.truecylonspices.com 3.1.3 Cinnamomum burmannii • Other names: • Korintje cassia • Padang cassia • Batavia cassia • Indonesian cinnamon • Origin: • Indonesia • Pros: • Cheap • Spicy Cinnamon flavor Image credit: Wikipedia • Cons: • High Coumarin Levels www.truecylonspices.com 3.2.1 b. Cinnamomum verum • Other names: • Cinnamomum zeylanicum (old Latin name) • True Cinnamon • Ceylon Cinnamon • Pure cinnamon • Mexican cinnamon • Sri Lanka cinnamon • Canela (Spanish for cinnamon) • Origin: • Sri Lanka (90%), • India, Madagascar, Brazil, Caribbean • Pros: • Ultra Low Coumarin levels • Softer and subtle taste • crumbly • Cons: • Expensive www.truecylonspices.com 4. Usage of Cinnamon • Usage of Cinnamon bark • As a spice. -

1 Chapter 1 Introduction 1.1 Statement and Significance
1 CHAPTER 1 INTRODUCTION 1.1 STATEMENT AND SIGNIFICANCE OF THE PROBLEM Nowadays most consumers are concerned about using chemical preservatives in foods to prevent lipid oxidation and the growth of spoiling microbes since some of these additives trend to be carcinogenic or can promote the growth of tumors, so that the decrease in chemical preservatives usage is of interest. Lipid oxidation and bacterial contamination are the main factors to determine food quality loss and shelf- life reduction; therefore, delaying lipid oxidation and preventing bacterial cross- contamination are highly important to food processors (Fernandez-Lopez et al., 2005). To inhibit lipid oxidation and microbial growth, the synthetic antioxidants have been widely used, the most common are butylated hydroxyanisole (BHA), butylated hydroxytoluene (BHT) and tert-butylated hydroxyquinone (TBHQ) as potential inhibitors for lipid peroxidation and thereby stabilize fat containing food- stuffs (Löliger, 1991). However, BHA and BHT were found to be anticarcinogenic as well as carcinogenic in experiments or animals and it has been established that they are involved in tumor formation (Botterweck et al., 2000). In recent years, the global demand of natural foods and natural preservatives being consumed for health are preferable. Using plant essential oils and their extracts, the new approach to prevent the proliferation of microorganism or protect food from oxidation, as alternative natural preservative agents is becoming popular due to their medicinal, antimicrobial and antioxidant properties (Pattnaik et al., 1997: Moreno et al., 2002: Sharififar et al., 2007: Al-Fatimi et al., 2007: Yang and Clausen, 2007). The antimicrobial and antioxidant activities of plant essential oils and their extracts are particularly beneficial for utilizations in the food industry such as in raw and processed food preservation, besides those herbal plants and dietary spices are widely cultivated and approved for consumption. -

Influence of Tea Tree Essential Oil and Poly(Ethylene Glycol)
materials Article Influence of Tea Tree Essential Oil and Poly(ethylene glycol) on Antibacterial and Physicochemical Properties of Polylactide-Based Films Iwona Tarach 1, Ewa Olewnik-Kruszkowska 1,* , Agnieszka Richert 2 , Magdalena Gierszewska 1 and Anna Rudawska 3 1 Chair of Physical Chemistry and Physicochemistry of Polymers, Faculty of Chemistry, Nicolaus Copernicus University in Toru´n,Gagarina 7 Street, 87-100 Toru´n,Poland; [email protected] (I.T.); [email protected] (M.G.) 2 Chair of Genetics, Faculty of Biological and Veterinary Sciences, Nicolaus Copernicus University in Toru´n, Lwowska 1 Street, 87-100 Toru´n,Poland; [email protected] 3 Department of Production Engineering, Faculty of Mechanical Engineering, Lublin University of Technology, 20-618 Lublin, Poland; [email protected] * Correspondence: [email protected]; Tel.: +48-56-611-2210 Received: 5 October 2020; Accepted: 1 November 2020; Published: 4 November 2020 Abstract: The aim of the study was to establish the influence of poly(ethylene glycol) (PEG) on the properties of potential biodegradable packaging materials with antibacterial properties, based on polylactide (PLA) and tea tree essential oil (TTO). The obtained polymeric films consisted of PLA, a natural biocide, and tea tree essential oil (5–20 wt. %) was prepared with or without an addition of 5 wt. % PEG. The PLA-based materials have been tested, taking into account their morphology, and their thermal, mechanical and antibacterial properties against Staphylococcus aureus and Escherichia coli. It was established that the introduction of a plasticizer into the PLA–TTO systems leads to an increase in tensile strength, resistance to deformation, as well an increased thermal stability, in comparison to films modified using only TTO. -

Melissa Officinalis L., a Valuable Medicine Plant: a Review
Journal of Medicinal Plants Research Vol. 4(25), pp. 2753-2759, 29 December Special Review, 2010 Available online at http://www.academicjournals.org/JMPR ISSN 1996-0875 ©2010 Academic Journals Review Melissa officinalis L., a valuable medicine plant: A review Moradkhani H.1, Sargsyan E.1, Bibak H.2, Naseri B.3, Sadat-Hosseini M.2, Fayazi-Barjin A.4 and Meftahizade H.5* 1Institute of Hydroponic Problems, National Academic of Sciences, Yerevan, Republic of Armenia. 2Department of plant production, faculty of Agriculture, university of Jiroft, Kerman, Iran. 3Faculty of Islamic Azad University, Ilam, Iran. 4Department of Plant Protection, University of Tehran, Iran. 5Researcher of ACECR Medicinal Plants Center, Ilam, Iran. Accepted 6 December, 2010 Melissa officinalis L., a valuable medicinal plant in herbal medicine is native to the eastern Mediterranean Region and western Asia. The constituent of the essential oil of the plant in various climates is different, but citral (geranial and neral), citronellal, geraniol are main components. Many parameters influencing essential oil composition and yield, such as light intensity, nutrient, temperature, cultural practice genotype, plant part age, harvesting time. Lemon balm has been traditionally used for different medical purposes as tonic, antispasmodic, carminative, diaphoretic, surgical dressing for wounds, sedative-hypnotic strengthening the memory, and relief of stress induced headache, but in modern pharmacology is value in the management of mild to moderate Alzheimer’s, against migraine and rheumatism, antitumel and antioxidant activities. Key words: Melissa officinalis, essential oil, pharmacology and antioxidant. INTRODUCTION Lemon balm, member of the family Lamiaceae (formerly years may no longer germinate (Zargari, 1991). Labiatae) is a perennial bushy plant and is upright, Lemon balm has a hairy root system with many lateral reaching a height of about 1 m. -

Essential Oils for Beginners Essential Oils for Beginners
Essential Oils for Beginners Essential Oils for Beginners Introduction The Power of the Entire Earth in One Bottle Our planet is a complicated system, made up of intricate ecosystems, endless molecules, and powerful elements. Over the span of 4.5 billion years, the Earth has formed colossal mountains, green valleys, leafy jungles, and vast forests, providing a home for countless plants and animals. On the 196,900,000 square miles of the planet’s surface, scientists estimate that there are 390,000 plants—with new species being discovered all the time. The hundreds of thousands of plants actually perform a number of important functions for the planet. Plants help control the climate, process carbon dioxide and release oxygen into the air to help us breathe, keep living organisms alive, and can be used for food and health solutions, among many other practical uses. With so many benefits, it is no surprise that plants have been used since the beginning of time to help humans perform everyday tasks. 2 Essential Oils for Beginners Ancient times People of ancient civilizations used entire plants, extracts, and various plant parts to make life easier, transforming the gifts of the earth into everything from textiles to remedies. Ancient people believed that the earth gave them everything they needed to solve all of their problems. Present day Fast-forward to modern day. Plants are still everywhere around us, yet with new inventions and technology, we’ve grown accustomed to using synthetic products rather than the gifts of nature to solve everyday challenges. Technology has made life easier, but along with technological advancements, we’ve seen a decline in many areas of quality of life. -
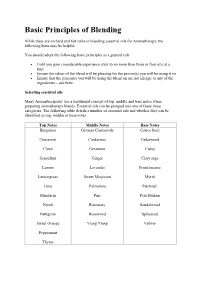
Basic Principles of Blending
Basic Principles of Blending While there are no hard and fast rules in blending essential oils for Aromatherapy, the following hints may be helpful. You should adopt the following basic principles as a general rule Until you gain considerable experience stick to no more than three or four oils at a time Ensure the odour of the blend will be pleasing for the person(s) you will be using it on Ensure that the person(s) you will be using the blend on are not allergic to any of the ingredients – ask them Selecting essential oils Many Aromatherapists’ use a traditional concept of top, middle and base notes when preparing aromatherapy blends. Essential oils can be grouped into one of these three categories. The following table details a number of essential oils and whether they can be identified as top, middle or base notes. Top Notes Middle Notes Base Notes Bergamot German Chamomile Carrot Seed Cinnamon Cardamom Cedarwood Clove Geranium Cistus Grapefruit Ginger Clary sage Lemon Lavender Frankincense Lemongrass Sweet Marjoram Myrrh Lime Palmarosa Patchouli Mandarin Pine Peru Balsam Neroli Rosemary Sandalwood Petitgrain Rosewood Spikenard Sweet Orange Ylang Ylang Vetiver Peppermint Thyme Top notes are usually fresh, citrus and light. These form the blend’s initial impression, giving brightness and clarity to it. They usually have refreshing and uplifting therapeutic properties. Middle notes (or heart notes) last longer, imparting the warmth and fullness of the blend. They give body to a blend and are mostly found in essential oils distilled from leaves and herbs. Base notes are the heavy smelling, deeply resonating and have a profound influence on the blend. -

American Version
COMPENDIUM OF INTERNATIONAL METHODS OF ANALYSIS-OIV Detection of preservatives and fermentation inhibitors Method OIV-MA-AS4-02E Type IV method Detection of preservatives and fermentation inhibitors Method A 35 modified by resolution Oeno 6/2006 1. Examination of dehydroacetic acid 1.1 Principle Wine acidified with sulfuric acid is extracted with a mixture of equal parts of diethyl ether and petroleum ether. After evaporation of the solvent, the extract, recovered with a small quantity of 96% ethanol (v/v) is deposited on a thin layer of polyamide and silica gel with fluorescent indicator and subjected to the action of the mobile solvent (benzene-acetone-acetic acid). The dehydroacetic acid is identified and characterized by ultraviolet examination of the chromatogram. 1.2 Apparatus 1.2.1 Equipment for thin layer chromatography 1.2.2 Oven 1.2.3 Rotary evaporator 1.2.4 UV lamp 254 nm. 1.3 Reagents 1.3.1 Diethyl ether 1.3.2 Petroleum ether (boiling point 40 °C) 1.3.3 Methanol 1.3.4 Sulfuric acid, 20% (v/v) 1.3.5 Anhydrous sodium sulfate. 1.3.6 Ethanol, 96% (v/v) . 1.3.7 Chromatographic separation layer: 10 g polyamide powder with fluorescent indicator(e.g. polyamide DC II UV254 from Macherey-Nagel) mixed vigorously with 60 mL methanol. Add while stirring, 10 ml of water and 10ml of silica gel (with fluorescent indicator), e.g. Kiesselgel GF254 Merck. Spread this mixture on 5 plates (200 x 200 mm) to a thickness of 0.25 mm. Dry the plates at room temperature for 30 minutes, then place in a 70°C oven for 10 min. -

SDS Contains All of the Information Required by the HPR
SAFETY DATA SHEET Preparation Date: 4/1/2014 Revision date 6/24/2019 Revision Number: G2 1. IDENTIFICATION Product identifier Product code: P1040 Product Name: PETROLEUM ETHER, B.R. 20 DEG -40 DEG C Other means of identification Synonyms: Benzine (light petroleum distillate) Benzoline Canadol Ligroin Painters naphtha Refined solvent naphtha CAS #: 8032-32-4 RTECS # OI6180000 CI#: Not available Recommended use of the chemical and restrictions on use Recommended use: Solvent. Uses advised against No information available Supplier: Spectrum Chemical Mfg. Corp 14422 South San Pedro St. Gardena, CA 90248 (310) 516-8000 Order Online At: https://www.spectrumchemical.com Emergency telephone number Chemtrec 1-800-424-9300 Contact Person: Tom Tyner (USA - West Coast) Contact Person: Ibad Tirmiz (USA - East Coast) 2. HAZARDS IDENTIFICATION Classification This chemical is considered hazardous by the 2012 OSHA Hazard Communication Standard (29 CFR 1910.1200) Considered a dangerous substance or mixture according to the Globally Harmonized System (GHS) Acute toxicity - Inhalation (Gases) Category 4 Acute toxicity - Inhalation (Vapors) Category 4 Skin corrosion/irritation Category 2 Serious eye damage/eye irritation Category 2A Specific target organ toxicity (single exposure) Category 3 Specific target organ toxicity (repeated exposure) Category 2 Aspiration toxicity Category 1 Flammable liquids Category 2 Label elements Product code: P1040 Product name: PETROLEUM ETHER, Page 1 / 13 B.R. 20 DEG -40 DEG C Danger Hazard statements Harmful if inhaled Causes -

Evaluation of Essential Oils and Extracts of Rose Geranium and Rose Petals As Natural Preservatives in Terms of Toxicity, Antimicrobial, and Antiviral Activity
pathogens Article Evaluation of Essential Oils and Extracts of Rose Geranium and Rose Petals as Natural Preservatives in Terms of Toxicity, Antimicrobial, and Antiviral Activity Chrysa Androutsopoulou 1, Spyridoula D. Christopoulou 2, Panagiotis Hahalis 3, Chrysoula Kotsalou 1, Fotini N. Lamari 2 and Apostolos Vantarakis 1,* 1 Department of Public Health, Faculty of Medicine, University of Patras, 26504 Patras, Greece; [email protected] (C.A.); [email protected] (C.K.) 2 Laboratory of Pharmacognosy & Chemistry of Natural Products, Department of Pharmacy, University of Patras, 26504 Patras, Greece; [email protected] (S.D.C.); [email protected] (F.N.L.) 3 Tentoura Castro-G.P. Hahalis Distillery, 26225 Patras, Greece; [email protected] * Correspondence: [email protected] Abstract: Essential oils (EOs) and extracts of rose geranium (Pelargonium graveolens) and petals of rose (Rosa damascena) have been fully characterized in terms of composition, safety, antimicrobial, and antiviral properties. They were analyzed against Escherichia coli, Salmonella enterica serovar Typhimurium, Staphylococcus aureus, Aspergillus niger, and Adenovirus 35. Their toxicity and life span were also determined. EO of P. graveolens (5%) did not retain any antibacterial activity (whereas at Citation: Androutsopoulou, C.; 100% it was greatly effective against E. coli), had antifungal activity against A. niger, and significant Christopoulou, S.D.; Hahalis, P.; antiviral activity. Rose geranium extract (dilutions 25−90%) (v/v) had antifungal and antibacterial Kotsalou, C.; Lamari, F.N.; Vantarakis, activity, especially against E. coli, and dose-dependent antiviral activity. Rose petals EO (5%) retains A. Evaluation of Essential Oils and low inhibitory activity against S. aureus and S. Typhimurium growth (about 20−30%), antifungal Extracts of Rose Geranium and Rose activity, and antiviral activity for medium to low virus concentrations. -

Health Benefits of Geranium Essential Oil
Health Benefits of Geranium Essential Oil The health benefits of Geranium Essential Oil can be attributed to its properties like astringent, haemostatic, cicatrisant, cytophylactic, diuretic, deodorant, styptic, tonic, vermifuge and vulnerary etc. The Essential Oil of Geranium is extracted through steam distillation of stem and leaves of Geranium plant, bearing scientific name Pelargonium Odorantissimum. The main components of this oil are Alpha Pinene, Myrcene, Limonene, Menthone, Linalool, Geranyl Acetate, Citronellol, Geraniol and Geranyl Butyrate. The Essential Oil of Geranium has a lot to offer in terms of health. We can benefit from the following properties of Geranium Oil. • Astringent: The main function of an astringent is to induce contractions. Accordingly Geranium Oil, being an astringent, makes the gums, muscles (including contraction of abdominal muscles which gives you a better look), intestines, skin, tissues and blood vessels to contract. This may be helpful in many ways. It can prevent muscles and skin from hanging loose, untimely loosening and fall of teeth, wrinkles and can even stop haemorrhage by contracting blood vessels. • Anti Bacterial & Anti Microbial: This property does not let bacteria or microbes develop on wounds and otherwise and keeps you safe from infections. • Haemostatic: Geranium Oil can stop haemorrhage in two ways. First, being an astringent (more specifically, a styptic), it causes contraction of blood vessels and helps stop flow of blood, as discussed above. Second, being a Haemostatic, it speeds up coagulation or clotting of blood. • Cicatrisant: Everybody wants that his or her skin to be free from scars and after marks of fat-cracks, surgeries, boils and acne or pox. -

Effects of Cinnamon Water Extract As a Cariostatic Agent
EFFECTS OF CINNAMON WATER EXTRACT AS A CARIOSTATIC AGENT ON NICOTINE-INDUCED STREPTOCOCCUS MUTANS BIOFILM by Abdulaziz Alshahrani Submitted to the Graduate Faculty of the School of Dentistry in partial fulfillment of the requirements for the degree of Master of Science in Dentistry, Indiana University School of Dentistry, March 2019. ii Thesis accepted by the faculty of the Department of Operative Dentistry, Indiana University, in partial fulfillment of the requirements for the degree of Master of Science in Dentistry. Frank Lippert Richard L. Gregory Chair of the Committee Norman Blaine Cook Program Director Date iii DEDICATION iv I dedicate this work to the Almighty Allah for his blessings, graces and virtues. In addition, I dedicate this work to my beloved parents, without whose prayers and support I would never have been who I am today, and to my lovely brothers and sisters for their love and encouragement during my studies. Lastly, I dedicate this work to my mentors and instructors throughout all my schooling. v ACKNOWLEDGMENTS vi My appreciation and thanks go to my research committee – Dr. Richard L. Gregory, Dr. Blaine Cook, and Dr. Frank Lippert – for their guidance and encouragement throughout the conduct of my research. Without their knowledge and thoughtful advice, this research project would never have been completed. I would like to extend thanks to Mr. George Eckert and Ms. Sruthi for their help in the biostatistics section. I am grateful to my amazing and loving parents for their endless support and encouragement. I would like to thank my wonderful brothers and sisters for their love and encouragement.