RNF43 Is Frequently Mutated in Colorectal and Endometrial Cancers
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Dual Regulation of Planar Polarization by Secreted Wnts and Vangl2 in the Developing Mouse Cochlea Elvis Huarcaya Najarro1, Jennifer Huang1, Adrian Jacobo2, Lee A
© 2020. Published by The Company of Biologists Ltd | Development (2020) 147, dev191981. doi:10.1242/dev.191981 RESEARCH ARTICLE Dual regulation of planar polarization by secreted Wnts and Vangl2 in the developing mouse cochlea Elvis Huarcaya Najarro1, Jennifer Huang1, Adrian Jacobo2, Lee A. Quiruz1, Nicolas Grillet1 and Alan G. Cheng1,* ABSTRACT on the other. Flamingo (Fmi/Celsr1, Fmi/Celsr2 and Fmi/Celsr3) is Planar cell polarity (PCP) proteins localize asymmetrically to instruct present on both poles of the cell. Defective PCP signaling cell polarity within the tissue plane, with defects leading to deformities represented by a lack of polarized PCP components leads to of the limbs, neural tube and inner ear. Wnt proteins are evolutionarily congenital heart and tracheal abnormalities, skeletal dysplasia, conserved polarity cues, yet Wnt mutants display variable PCP neural tube defects as well as cochlear deformities (Butler and defects; thus, how Wnts regulate PCP remains unresolved. Here, we Wallingford, 2017; White et al., 2018). Despite their crucial roles, have used the developing cochlea as a model system to show that our understanding of upstream signals orchestrating PCP signaling secreted Wnts regulate PCP through polarizing a specific subset of is rather limited. PCP proteins. Conditional deletion of Wntless or porcupine, both of Wnt proteins have been implicated as upstream polarity cues for which are essential for secretion of Wnts, caused misrotated sensory PCP signaling. For example, limb morphogenesis in mice requires a cells and shortened cochlea – both hallmarks of PCP defects. gradient of Wnt5a, which has been reported to act as an instructive Wntless-deficient cochleae lacked the polarized PCP components cue to establish PCP (Gao et al., 2018, 2011). -

Mutational Landscape Differences Between Young-Onset and Older-Onset Breast Cancer Patients Nicole E
Mealey et al. BMC Cancer (2020) 20:212 https://doi.org/10.1186/s12885-020-6684-z RESEARCH ARTICLE Open Access Mutational landscape differences between young-onset and older-onset breast cancer patients Nicole E. Mealey1 , Dylan E. O’Sullivan2 , Joy Pader3 , Yibing Ruan3 , Edwin Wang4 , May Lynn Quan1,5,6 and Darren R. Brenner1,3,5* Abstract Background: The incidence of breast cancer among young women (aged ≤40 years) has increased in North America and Europe. Fewer than 10% of cases among young women are attributable to inherited BRCA1 or BRCA2 mutations, suggesting an important role for somatic mutations. This study investigated genomic differences between young- and older-onset breast tumours. Methods: In this study we characterized the mutational landscape of 89 young-onset breast tumours (≤40 years) and examined differences with 949 older-onset tumours (> 40 years) using data from The Cancer Genome Atlas. We examined mutated genes, mutational load, and types of mutations. We used complementary R packages “deconstructSigs” and “SomaticSignatures” to extract mutational signatures. A recursively partitioned mixture model was used to identify whether combinations of mutational signatures were related to age of onset. Results: Older patients had a higher proportion of mutations in PIK3CA, CDH1, and MAP3K1 genes, while young- onset patients had a higher proportion of mutations in GATA3 and CTNNB1. Mutational load was lower for young- onset tumours, and a higher proportion of these mutations were C > A mutations, but a lower proportion were C > T mutations compared to older-onset tumours. The most common mutational signatures identified in both age groups were signatures 1 and 3 from the COSMIC database. -

The Role of Genetics Mutations in Genes PORCN, TWIST2, HCCS In
ISSN: 2378-3672 Asadi and Jamali. Int J Immunol Immunother 2019, 6:038 DOI: 10.23937/2378-3672/1410038 Volume 6 | Issue 1 International Journal of Open Access Immunology and Immunotherapy REVIEW ARTICLE The Role of Genetics Mutations in Genes PORCN, TWIST2, HCCS in Goltz Syndrome Shahin Asadi1* and Mahsa Jamali2 Check for updates Division of Medical Genetics and Molecular Pathology Research, Molecular Genetics-IRAN-TABRIZ, Iran *Corresponding author: Shahin Asadi, Division of Medical Genetics and Molecular Pathology Research, Molecular Genetics-IRAN-TABRIZ, Iran are usually formed around the nose, the lips, the anal Abstract holes and the genitals of women with Goltz syndrome. Goltz syndrome (focal skin hypoplasia) is a genetic disorder In addition, papillomas may be present in the throat, that primarily affects the skin, skeletal system, eyes and face. People with Goltz syndrome have birth defects. These especially in the esophagus or larynx, and can cause disorders include very thin skin veins (skin hypoplasia), pink swallowing, respiration or sleep poisoning. The papillo- yellow nodules, subcutaneous fat, lack of upper skin layers mas can be removed if necessary by surgery from the (aplasia cutis), small clusters of superficial skin vessels growing regions. People with Goltz syndrome may have (telangiectasia), and veins in dark skin Or bright. Goltz syndrome is caused by mutation genes PORCN, TWIST2, small and abnormal nails on the toes. In addition, hair in HCCS. the scalp of these people can be weak or fragile or not [2] (Figure 2). Keywords Goltz syndrome, PORCN, TWIST2, HCCS genes, Skin and Many people with Goltz syndrome also have hand Skeletal disorders and foot disorders, including abnormalities such as oligodactyly in fingers and toes, fingers and legs (cin- Generalizations of Goltz Syndrome dactyly) or ecd rhodactyly. -

Based on Network Pharmacology Tools to Investigate the Molecular Mechanism of Cordyceps Sinensis on the Treatment of Diabetic Nephropathy
Hindawi Journal of Diabetes Research Volume 2021, Article ID 8891093, 12 pages https://doi.org/10.1155/2021/8891093 Research Article Based on Network Pharmacology Tools to Investigate the Molecular Mechanism of Cordyceps sinensis on the Treatment of Diabetic Nephropathy Yan Li,1 Lei Wang,2 Bojun Xu,1 Liangbin Zhao,1 Li Li,1 Keyang Xu,3 Anqi Tang,1 Shasha Zhou,1 Lu Song,1 Xiao Zhang,1 and Huakui Zhan 1 1Hospital of Chengdu University of Traditional Chinese Medicine, Chengdu, 610072 Sichuan, China 2Key Laboratory of Chinese Internal Medicine of Ministry of Education and Dongzhimen Hospital, Beijing University of Chinese Medicine, Beijing 100700, China 3Zhejiang Chinese Medical University, Hangzhou, 310053 Zhejiang, China Correspondence should be addressed to Huakui Zhan; [email protected] Received 27 August 2020; Revised 17 January 2021; Accepted 24 January 2021; Published 8 February 2021 Academic Editor: Michaelangela Barbieri Copyright © 2021 Yan Li et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Background. Diabetic nephropathy (DN) is one of the most common complications of diabetes mellitus and is a major cause of end- stage kidney disease. Cordyceps sinensis (Cordyceps, Dong Chong Xia Cao) is a widely applied ingredient for treating patients with DN in China, while the molecular mechanisms remain unclear. This study is aimed at revealing the therapeutic mechanisms of Cordyceps in DN by undertaking a network pharmacology analysis. Materials and Methods. In this study, active ingredients and associated target proteins of Cordyceps sinensis were obtained via Traditional Chinese Medicine Systems Pharmacology Database (TCMSP) and Swiss Target Prediction platform, then reconfirmed by using PubChem databases. -

A Uniform Human Wnt Expression Library Reveals a Shared Secretory Pathway and Unique Signaling Activities
Differentiation 84 (2012) 203–213 Contents lists available at SciVerse ScienceDirect Differentiation journal homepage: www.elsevier.com/locate/diff A uniform human Wnt expression library reveals a shared secretory pathway and unique signaling activities Rani Najdi a, Kyle Proffitt b, Stephanie Sprowl a, Simran Kaur b, Jia Yu b, Tracy M. Covey b, David M. Virshup b,1, Marian L. Waterman a,n a Department of Microbiology and Molecular Genetics, University of California, Irvine, CA, USA b Program in Cancer and Stem Cell Biology, Duke-NUS Graduate Medical School, Singapore article info abstract Article history: Wnt ligands are secreted morphogens that control multiple developmental processes during embry- Received 24 April 2012 ogenesis and adult homeostasis. A diverse set of receptors and signals have been linked to individual Accepted 16 June 2012 Wnts, but the lack of tools for comparative analysis has limited the ability to determine which of these Available online 9 July 2012 signals are general for the entire Wnt family, and which define subsets of differently acting ligands. We Keywords: have created a versatile Gateway library of clones for all 19 human Wnts. An analysis comparing Wnt signaling epitope-tagged and untagged versions of each ligand shows that despite their similar expression at the b-catenin mRNA level, Wnts exhibit considerable variation in stability, processing and secretion. At least 14 out of Wnt modification and secretion the 19 Wnts activate b-catenin-dependent signaling, an activity that is cell type-dependent and tracks with the stabilization of b-catenin and LRP6 phosphorylation. We find that the core Wnt modification and secretion proteins Porcupine (PORCN) and Wntless (WLS) are essential for all Wnts to signal through b-catenin-dependent and independent pathways. -

Ncomms3787.Pdf
ARTICLE Received 6 Aug 2013 | Accepted 17 Oct 2013 | Published 14 Nov 2013 DOI: 10.1038/ncomms3787 OPEN Structural and molecular basis of ZNRF3/RNF43 transmembrane ubiquitin ligase inhibition by the Wnt agonist R-spondin Matthias Zebisch1, Yang Xu2,3, Christos Krastev1, Bryan T. MacDonald2, Maorong Chen2, Robert J.C. Gilbert1,XiHe2 & E. Yvonne Jones1 The four R-spondin (Rspo) proteins are secreted agonists of Wnt signalling in vertebrates, functioning in embryogenesis and adult stem cell biology. Through ubiquitination and degradation of Wnt receptors, the transmembrane E3 ubiquitin ligase ZNRF3 and related RNF43 antagonize Wnt signalling. Rspo ligands have been reported to inhibit the ligase activity through direct interaction with ZNRF3 and RNF43. Here we report multiple crystal structures of the ZNRF3 ectodomain (ZNRF3ecto), a signalling-competent Furin1–Furin2 (Fu1–Fu2) fragment of Rspo2 (Rspo2Fu1–Fu2), and Rspo2Fu1–Fu2 in complex with ZNRF3ecto,or RNF43ecto. A prominent loop in Fu1 clamps into equivalent grooves in the ZNRF3ecto and RNF43ecto surface. Rspo binding enhances dimerization of ZNRF3ecto but not of RNF43ecto. Comparison of the four Rspo proteins, mutants and chimeras in biophysical and cellular assays shows that their signalling potency depends on their ability to recruit ZNRF3 or RNF43 via Fu1 into a complex with LGR receptors, which interact with Rspo via Fu2. 1 Division of Structural Biology, Wellcome Trust Centre for Human Genetics, University of Oxford, Oxford OX3 7BN, UK. 2 F.M. Kirby Neurobiology Center, Department of Neurology, Boston Children’s Hospital, Harvard Medical School, Boston, Massachusetts 02115, USA. 3 Key Laboratory for Molecular Enzymology and Engineering of Ministry of Education, College of Life Science, Jilin University, Changchun 130012, China. -

Tumour-Stroma Signalling in Cancer Cell Motility and Metastasis
Tumour-Stroma Signalling in Cancer Cell Motility and Metastasis by Valbona Luga A thesis submitted in conformity with the requirements for the degree of Doctor of Philosophy, Department of Molecular Genetics, University of Toronto © Copyright by Valbona Luga, 2013 Tumour-Stroma Signalling in Cancer Cell Motility and Metastasis Valbona Luga Doctor of Philosophy Department of Molecular Genetics University of Toronto 2013 Abstract The tumour-associated stroma, consisting of fibroblasts, inflammatory cells, vasculature and extracellular matrix proteins, plays a critical role in tumour growth, but how it regulates cancer cell migration and metastasis is poorly understood. The Wnt-planar cell polarity (PCP) pathway regulates convergent extension movements in vertebrate development. However, it is unclear whether this pathway also functions in cancer cell migration. In addition, the factors that mobilize long-range signalling of Wnt morphogens, which are tightly associated with the plasma membrane, have yet to be completely characterized. Here, I show that fibroblasts secrete membrane microvesicles of endocytic origin, termed exosomes, which promote tumour cell protrusive activity, motility and metastasis via the exosome component Cd81. In addition, I demonstrate that fibroblast exosomes activate autocrine Wnt-PCP signalling in breast cancer cells as detected by the association of Wnt with Fzd receptors and the asymmetric distribution of Fzd-Dvl and Vangl-Pk complexes in exosome-stimulated cancer cell protrusive structures. Moreover, I show that Pk expression in breast cancer cells is essential for fibroblast-stimulated cancer cell metastasis. Lastly, I reveal that trafficking in cancer cells promotes tethering of autocrine Wnt11 to fibroblast exosomes. These studies further our understanding of the role of ii the tumour-associated stroma in cancer metastasis and bring us closer to a more targeted approach for the treatment of cancer spread. -
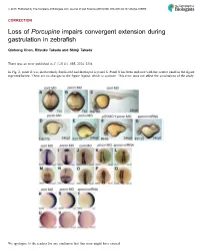
Porcupine Impairs Convergent Extension During Gastrulation in Zebrafish
ß 2015. Published by The Company of Biologists Ltd | Journal of Cell Science (2015) 000, 000–000 doi:10.1242/jcs.168989 CORRECTION Loss of Porcupine impairs convergent extension during gastrulation in zebrafish Qiuhong Chen, Ritsuko Takada and Shinji Takada There was an error published in J. Cell Sci. 125, 2224–2234. In Fig. 2, panel R was inadvertently duplicated and displayed as panel S. Panel S has been replaced with the correct panel in the figure reprinted below. There are no changes to the figure legend, which is accurate. This error does not affect the conclusions of the study. We apologise to the readers for any confusion that this error might have caused. 1 Journal of Cell Science JCS168989.3d 22/1/15 10:57:22 The Charlesworth Group, Wakefield +44(0)1924 369598 - Rev 9.0.225/W (Oct 13 2006) 2224 Research Article Loss of Porcupine impairs convergent extension during gastrulation in zebrafish Qiuhong Chen1, Ritsuko Takada1 and Shinji Takada1,2,* 1Okazaki Institute for Integrative Bioscience and National Institute for Basic Biology, National Institutes of Natural Sciences, Okazaki, Aichi 444-8787, Japan 2Department of Molecular Biomechanics, The Graduate University for Advanced Studies (SOKENDAI), Okazaki, Aichi 444-8787, Japan *Author for correspondence ([email protected]) Accepted 9 January 2012 Journal of Cell Science 125, 2224–2234 ß 2012. Published by The Company of Biologists Ltd doi: 10.1242/jcs.098368 Summary Porcupine (Porcn), an O-acyltransferase located in the endoplasmic reticulum (ER), is required for lipidation of Wnt proteins to enable their trafficking from the ER in mammalian cell culture. -

Microrna-124-3P Suppresses Mouse Lip Mesenchymal Cell Proliferation
Suzuki et al. BMC Genomics (2019) 20:852 https://doi.org/10.1186/s12864-019-6238-4 RESEARCH ARTICLE Open Access MicroRNA-124-3p suppresses mouse lip mesenchymal cell proliferation through the regulation of genes associated with cleft lip in the mouse Akiko Suzuki1,2, Hiroki Yoshioka1,2, Dima Summakia1, Neha G. Desai1,3, Goo Jun3,4, Peilin Jia5, David S. Loose4,6, Kenichi Ogata1,2, Mona V. Gajera1,3, Zhongming Zhao3,4,5 and Junichi Iwata1,2,4* Abstract Background: Cleft lip (CL), one of the most common congenital birth defects, shows considerable geographic and ethnic variation, with contribution of both genetic and environmental factors. Mouse genetic studies have identified several CL-associated genes. However, it remains elusive how these CL-associated genes are regulated and involved in CL. Environmental factors may regulate these genes at the post-transcriptional level through the regulation of non-coding microRNAs (miRNAs). In this study, we sought to identify miRNAs associated with CL in mice. Results: Through a systematic literature review and a Mouse Genome Informatics (MGI) database search, we identified 55 genes that were associated with CL in mice. Subsequent bioinformatic analysis of these genes predicted that a total of 33 miRNAs target multiple CL-associated genes, with 20 CL-associated genes being potentially regulated by multiple miRNAs. To experimentally validate miRNA function in cell proliferation, we conducted cell proliferation/viability assays for the selected five candidate miRNAs (miR-124-3p, let-7a-5p, let-7b-5p, let-7c-5p, and let-7d-5p). Overexpression of miR-124-3p, but not of the others, inhibited cell proliferation through suppression of CL-associated genes in cultured mouse embryonic lip mesenchymal cells (MELM cells) isolated from the developing mouse lip region. -

Porcn (NM 023638) Mouse Tagged ORF Clone Lentiviral Particle Product Data
OriGene Technologies, Inc. 9620 Medical Center Drive, Ste 200 Rockville, MD 20850, US Phone: +1-888-267-4436 [email protected] EU: [email protected] CN: [email protected] Product datasheet for MR226862L3V Porcn (NM_023638) Mouse Tagged ORF Clone Lentiviral Particle Product data: Product Type: Lentiviral Particles Product Name: Porcn (NM_023638) Mouse Tagged ORF Clone Lentiviral Particle Symbol: Porcn Synonyms: 2410004O13Rik; AW045557; DXHXS7465e; Mg61; mMg61; Mporc; porc; Ppn Vector: pLenti-C-Myc-DDK-P2A-Puro (PS100092) ACCN: NM_023638 ORF Size: 1383 bp ORF Nucleotide The ORF insert of this clone is exactly the same as(MR226862). Sequence: OTI Disclaimer: The molecular sequence of this clone aligns with the gene accession number as a point of reference only. However, individual transcript sequences of the same gene can differ through naturally occurring variations (e.g. polymorphisms), each with its own valid existence. This clone is substantially in agreement with the reference, but a complete review of all prevailing variants is recommended prior to use. More info OTI Annotation: This clone was engineered to express the complete ORF with an expression tag. Expression varies depending on the nature of the gene. RefSeq: NM_023638.3 RefSeq Size: 1896 bp RefSeq ORF: 1386 bp Locus ID: 53627 UniProt ID: Q9JJJ7 Gene Summary: Protein-serine O-palmitoleoyltransferase that acts as a key regulator of the Wnt signaling pathway by mediating the attachment of palmitoleate, a 16-carbon monounsaturated fatty acid (C16:1), to Wnt proteins. Serine palmitoleylation of WNT proteins is required for efficient binding to frizzled receptors.[UniProtKB/Swiss-Prot Function] This product is to be used for laboratory only. -

Downloaded Per Proteome Cohort Via the Web- Site Links of Table 1, Also Providing Information on the Deposited Spectral Datasets
www.nature.com/scientificreports OPEN Assessment of a complete and classifed platelet proteome from genome‑wide transcripts of human platelets and megakaryocytes covering platelet functions Jingnan Huang1,2*, Frauke Swieringa1,2,9, Fiorella A. Solari2,9, Isabella Provenzale1, Luigi Grassi3, Ilaria De Simone1, Constance C. F. M. J. Baaten1,4, Rachel Cavill5, Albert Sickmann2,6,7,9, Mattia Frontini3,8,9 & Johan W. M. Heemskerk1,9* Novel platelet and megakaryocyte transcriptome analysis allows prediction of the full or theoretical proteome of a representative human platelet. Here, we integrated the established platelet proteomes from six cohorts of healthy subjects, encompassing 5.2 k proteins, with two novel genome‑wide transcriptomes (57.8 k mRNAs). For 14.8 k protein‑coding transcripts, we assigned the proteins to 21 UniProt‑based classes, based on their preferential intracellular localization and presumed function. This classifed transcriptome‑proteome profle of platelets revealed: (i) Absence of 37.2 k genome‑ wide transcripts. (ii) High quantitative similarity of platelet and megakaryocyte transcriptomes (R = 0.75) for 14.8 k protein‑coding genes, but not for 3.8 k RNA genes or 1.9 k pseudogenes (R = 0.43–0.54), suggesting redistribution of mRNAs upon platelet shedding from megakaryocytes. (iii) Copy numbers of 3.5 k proteins that were restricted in size by the corresponding transcript levels (iv) Near complete coverage of identifed proteins in the relevant transcriptome (log2fpkm > 0.20) except for plasma‑derived secretory proteins, pointing to adhesion and uptake of such proteins. (v) Underrepresentation in the identifed proteome of nuclear‑related, membrane and signaling proteins, as well proteins with low‑level transcripts. -

Expanding the Phenotypic Spectrum of PORCN Variants in Two Males with Syndromic Microphthalmia
European Journal of Human Genetics (2015) 23, 551–554 & 2015 Macmillan Publishers Limited All rights reserved 1018-4813/15 www.nature.com/ejhg SHORT REPORT Expanding the phenotypic spectrum of PORCN variants in two males with syndromic microphthalmia Paul D Brady1, Hilde Van Esch1, Nathalie Fieremans1,GuyFroyen1, Anne Slavotinek2, Jan Deprest3,4, Koenraad Devriendt1 and Joris R Vermeesch*,1 Variants in PORCN are a cause of Goltz-Gorlin syndrome or Focal Dermal Hypoplasia, an X-linked dominant disorder affecting heterozygous females and until now considered to be embryonic lethal in males. Exome sequencing was performed in a family in which two male siblings were characterized by microphthalmia and additional congenital anomalies including diaphragmatic hernia, spina bifida and cardiac defects. Surprisingly, we identified a maternally inherited variant in PORCN present in both males as well as in two female siblings. This represents the first finding of a PORCN variant in non-mosaic males affected with Goltz-Gorlin syndrome. The apparently asymptomatic mother showed extreme skewing of X-inactivation (90%), an asymptomatic female sibling showed skewing of 88%, and the second female sibling affected with cutis aplasia of the scalp showed X-inactivation considered within the normal range. European Journal of Human Genetics (2015) 23, 551–554; doi:10.1038/ejhg.2014.135; published online 16 July 2014 INTRODUCTION The skin was normal and no other digital or skeletal abnormalities were Microphthalmia occurs with an incidence of 0.9–2.3 per 10 000,1 and observed. He was deceased at day 0, with confirmation of the clinical findings is estimated to occur in association with additional congenital by pathology examination.