The Role of the Microglial Cx3cr1 Pathway in the Postnatal Maturation of Retinal Photoreceptors
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Synergistic Genetic Interactions Between Pkhd1 and Pkd1 Result in an ARPKD-Like Phenotype in Murine Models
BASIC RESEARCH www.jasn.org Synergistic Genetic Interactions between Pkhd1 and Pkd1 Result in an ARPKD-Like Phenotype in Murine Models Rory J. Olson,1 Katharina Hopp ,2 Harrison Wells,3 Jessica M. Smith,3 Jessica Furtado,1,4 Megan M. Constans,3 Diana L. Escobar,3 Aron M. Geurts,5 Vicente E. Torres,3 and Peter C. Harris 1,3 Due to the number of contributing authors, the affiliations are listed at the end of this article. ABSTRACT Background Autosomal recessive polycystic kidney disease (ARPKD) and autosomal dominant polycystic kidney disease (ADPKD) are genetically distinct, with ADPKD usually caused by the genes PKD1 or PKD2 (encoding polycystin-1 and polycystin-2, respectively) and ARPKD caused by PKHD1 (encoding fibrocys- tin/polyductin [FPC]). Primary cilia have been considered central to PKD pathogenesis due to protein localization and common cystic phenotypes in syndromic ciliopathies, but their relevance is questioned in the simple PKDs. ARPKD’s mild phenotype in murine models versus in humans has hampered investi- gating its pathogenesis. Methods To study the interaction between Pkhd1 and Pkd1, including dosage effects on the phenotype, we generated digenic mouse and rat models and characterized and compared digenic, monogenic, and wild-type phenotypes. Results The genetic interaction was synergistic in both species, with digenic animals exhibiting pheno- types of rapidly progressive PKD and early lethality resembling classic ARPKD. Genetic interaction be- tween Pkhd1 and Pkd1 depended on dosage in the digenic murine models, with no significant enhancement of the monogenic phenotype until a threshold of reduced expression at the second locus was breached. -

A Computational Approach for Defining a Signature of Β-Cell Golgi Stress in Diabetes Mellitus
Page 1 of 781 Diabetes A Computational Approach for Defining a Signature of β-Cell Golgi Stress in Diabetes Mellitus Robert N. Bone1,6,7, Olufunmilola Oyebamiji2, Sayali Talware2, Sharmila Selvaraj2, Preethi Krishnan3,6, Farooq Syed1,6,7, Huanmei Wu2, Carmella Evans-Molina 1,3,4,5,6,7,8* Departments of 1Pediatrics, 3Medicine, 4Anatomy, Cell Biology & Physiology, 5Biochemistry & Molecular Biology, the 6Center for Diabetes & Metabolic Diseases, and the 7Herman B. Wells Center for Pediatric Research, Indiana University School of Medicine, Indianapolis, IN 46202; 2Department of BioHealth Informatics, Indiana University-Purdue University Indianapolis, Indianapolis, IN, 46202; 8Roudebush VA Medical Center, Indianapolis, IN 46202. *Corresponding Author(s): Carmella Evans-Molina, MD, PhD ([email protected]) Indiana University School of Medicine, 635 Barnhill Drive, MS 2031A, Indianapolis, IN 46202, Telephone: (317) 274-4145, Fax (317) 274-4107 Running Title: Golgi Stress Response in Diabetes Word Count: 4358 Number of Figures: 6 Keywords: Golgi apparatus stress, Islets, β cell, Type 1 diabetes, Type 2 diabetes 1 Diabetes Publish Ahead of Print, published online August 20, 2020 Diabetes Page 2 of 781 ABSTRACT The Golgi apparatus (GA) is an important site of insulin processing and granule maturation, but whether GA organelle dysfunction and GA stress are present in the diabetic β-cell has not been tested. We utilized an informatics-based approach to develop a transcriptional signature of β-cell GA stress using existing RNA sequencing and microarray datasets generated using human islets from donors with diabetes and islets where type 1(T1D) and type 2 diabetes (T2D) had been modeled ex vivo. To narrow our results to GA-specific genes, we applied a filter set of 1,030 genes accepted as GA associated. -

Cep78 Is a New Centriolar Protein Involved in Plk4-Induced Centriole
© 2016. Published by The Company of Biologists Ltd | Journal of Cell Science (2016) 129, 2713-2718 doi:10.1242/jcs.184093 SHORT REPORT Cep78 is a new centriolar protein involved in Plk4-induced centriole overduplication Kathrin Brunk1,§, Mei Zhu1,*,§, Felix Bärenz1, Anne-Sophie Kratz1, Uta Haselmann-Weiss2, Claude Antony2,‡ and Ingrid Hoffmann1,¶ ABSTRACT 2006; Brito et al., 2012). Major components of the pathway in Centrioles are core components of centrosomes, the major human cells are the serine/threonine polo-like kinase 4 (Plk4), microtubule-organizing centers of animal cells, and act as basal Cep192, Cep152, Sas-6, STIL and CPAP (also known as CENPJ) bodies for cilia formation. Control of centriole number is therefore (Brito et al., 2012). In human cells, Plk4, Sas-6 and STIL localize to crucial for genome stability and embryogenesis. Centriole duplication the sites of procentriole formation and collaborate to induce requires the serine/threonine protein kinase Plk4. Here, we identify cartwheel assembly during daughter centriole formation. Plk4 is a Cep78 as a human centrosomal protein and a new interaction partner structurally divergent polo-like kinase family member as it harbors – of Plk4. Cep78 is mainly a centriolar protein that localizes to the three polo-boxes (PB1 PB3) whereas Plk1, Plk2 and Plk3 only centriolar wall. Furthermore, we find that Plk4 binds to Cep78 through have two polo-boxes, PB1 and PB2 (Slevin et al., 2012). its N-terminal domain but that Cep78 is not an in vitro Plk4 substrate. Few substrates of Plk4 have been described to date, including Cep78 colocalizes with Plk4 at centrioles and is required for STIL (Ohta et al., 2014; Dzhindzhev et al., 2014; Kratz et al., 2015; Plk4-induced centriole overduplication. -

The Transformation of the Centrosome Into the Basal Body: Similarities and Dissimilarities Between Somatic and Male Germ Cells and Their Relevance for Male Fertility
cells Review The Transformation of the Centrosome into the Basal Body: Similarities and Dissimilarities between Somatic and Male Germ Cells and Their Relevance for Male Fertility Constanza Tapia Contreras and Sigrid Hoyer-Fender * Göttingen Center of Molecular Biosciences, Johann-Friedrich-Blumenbach Institute for Zoology and Anthropology-Developmental Biology, Faculty of Biology and Psychology, Georg-August University of Göttingen, 37077 Göttingen, Germany; [email protected] * Correspondence: [email protected] Abstract: The sperm flagellum is essential for the transport of the genetic material toward the oocyte and thus the transmission of the genetic information to the next generation. During the haploid phase of spermatogenesis, i.e., spermiogenesis, a morphological and molecular restructuring of the male germ cell, the round spermatid, takes place that includes the silencing and compaction of the nucleus, the formation of the acrosomal vesicle from the Golgi apparatus, the formation of the sperm tail, and, finally, the shedding of excessive cytoplasm. Sperm tail formation starts in the round spermatid stage when the pair of centrioles moves toward the posterior pole of the nucleus. The sperm tail, eventually, becomes located opposed to the acrosomal vesicle, which develops at the anterior pole of the nucleus. The centriole pair tightly attaches to the nucleus, forming a nuclear membrane indentation. An Citation: Tapia Contreras, C.; articular structure is formed around the centriole pair known as the connecting piece, situated in the Hoyer-Fender, S. The Transformation neck region and linking the sperm head to the tail, also named the head-to-tail coupling apparatus or, of the Centrosome into the Basal in short, HTCA. -

Suppl. Table 1
Suppl. Table 1. SiRNA library used for centriole overduplication screen. Entrez Gene Id NCBI gene symbol siRNA Target Sequence 1070 CETN3 TTGCGACGTGTTGCTAGAGAA 1070 CETN3 AAGCAATAGATTATCATGAAT 55722 CEP72 AGAGCTATGTATGATAATTAA 55722 CEP72 CTGGATGATTTGAGACAACAT 80071 CCDC15 ACCGAGTAAATCAACAAATTA 80071 CCDC15 CAGCAGAGTTCAGAAAGTAAA 9702 CEP57 TAGACTTATCTTTGAAGATAA 9702 CEP57 TAGAGAAACAATTGAATATAA 282809 WDR51B AAGGACTAATTTAAATTACTA 282809 WDR51B AAGATCCTGGATACAAATTAA 55142 CEP27 CAGCAGATCACAAATATTCAA 55142 CEP27 AAGCTGTTTATCACAGATATA 85378 TUBGCP6 ACGAGACTACTTCCTTAACAA 85378 TUBGCP6 CACCCACGGACACGTATCCAA 54930 C14orf94 CAGCGGCTGCTTGTAACTGAA 54930 C14orf94 AAGGGAGTGTGGAAATGCTTA 5048 PAFAH1B1 CCCGGTAATATCACTCGTTAA 5048 PAFAH1B1 CTCATAGATATTGAACAATAA 2802 GOLGA3 CTGGCCGATTACAGAACTGAA 2802 GOLGA3 CAGAGTTACTTCAGTGCATAA 9662 CEP135 AAGAATTTCATTCTCACTTAA 9662 CEP135 CAGCAGAAAGAGATAAACTAA 153241 CCDC100 ATGCAAGAAGATATATTTGAA 153241 CCDC100 CTGCGGTAATTTCCAGTTCTA 80184 CEP290 CCGGAAGAAATGAAGAATTAA 80184 CEP290 AAGGAAATCAATAAACTTGAA 22852 ANKRD26 CAGAAGTATGTTGATCCTTTA 22852 ANKRD26 ATGGATGATGTTGATGACTTA 10540 DCTN2 CACCAGCTATATGAAACTATA 10540 DCTN2 AACGAGATTGCCAAGCATAAA 25886 WDR51A AAGTGATGGTTTGGAAGAGTA 25886 WDR51A CCAGTGATGACAAGACTGTTA 55835 CENPJ CTCAAGTTAAACATAAGTCAA 55835 CENPJ CACAGTCAGATAAATCTGAAA 84902 CCDC123 AAGGATGGAGTGCTTAATAAA 84902 CCDC123 ACCCTGGTTGTTGGATATAAA 79598 LRRIQ2 CACAAGAGAATTCTAAATTAA 79598 LRRIQ2 AAGGATAATATCGTTTAACAA 51143 DYNC1LI1 TTGGATTTGTCTATACATATA 51143 DYNC1LI1 TAGACTTAGTATATAAATACA 2302 FOXJ1 CAGGACAGACAGACTAATGTA -

Plk4-Induced Centriole Biogenesis in Human Cells
Plk4-induced Centriole Biogenesis in Human Cells Dissertation zur Erlangung des Doktorgrades der Naturwissenschaften der Fakultät für Biologie der Ludwig-Maximilians Universität München Vorgelegt von Julia Kleylein-Sohn München, 2007 Dissertation eingereicht am: 27.11.2007 Tag der mündlichen Prüfung: 18.04.2008 Erstgutachter: Prof. E. A. Nigg Zweitgutachter: PD Dr. Angelika Böttger 2 Hiermit erkläre ich, dass ich die vorliegende Dissertation selbständig und ohne unerlaubte Hilfe angefertigt habe. Sämtliche Experimente wurden von mir selbst durchgeführt, soweit nicht explizit auf Dritte verwiesen wird. Ich habe weder an anderer Stelle versucht, eine Dissertation oder Teile einer solchen einzureichen bzw. einer Prüfungskommission vorzulegen, noch eine Doktorprüfung zu absolvieren. München, den 22.11.2007 3 TABLE OF CONTENTS SUMMARY…………………………………………………………………………..………. 6 INTRODUCTION……………………………………………………………………………. 7 Structure of the centrosome…………………………………………………………….. 7 The centrosome cycle…………………………………………………………………..10 Kinases involved in the regulation of centriole duplication………………………….12 Maintenance of centrosome numbers………………………………………………...13 Licensing of centriole duplication……………………………………………………... 15 ‘De novo ’ centriole assembly pathways in mammalian cells…………………..…...15 Templated centriole biogenesis in mammalian cells……………………………….. 18 The role of centrins and Sfi1p in centrosome duplication ……………………...…..19 Centriole biogenesis in C. elegans …………………………………………………… 21 Centriole biogenesis in human cells………………………………………………….. 23 Centrosome -
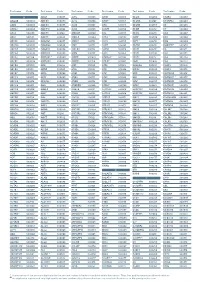
Aagab S00002 Aars S00003 Aars2 S00004 Aass S02483
Test name Code Test name Code Test name Code Test name Code Test name Code Test name Code A ADAR S00053 ALPL S00105 ARSB S00153 BCL10 S02266 C5AR2 S00263 AAGAB S00002 ADCK3 S00054 ALS2 S00106 ARSE * S00154 BCL11A S02167 C5ORF42 S00264 AARS S00003 ADCK4 S00055 ALX3 S00107 ARX S00155 BCL11B S02358 C6 S00265 AARS2 S00004 ADCY10 S02094 ALX4 S00108 ASAH1 S00156 BCOR S00212 C7 S00266 AASS S02483 ADCY3 S02184 AMACR S00109 ASL S00157 BCS1L S00213 C8A S00267 ABAT S02191 ADCY5 S02226 AMELX S02289 ASNS * S02508 BDNF S02509 C8B S00268 ABCA1 S00005 ADGRG1 S00057 AMER1 S00110 ASPA S00158 BDP1 * S00214 C8G S00269 ABCA12 S00006 ADGRG6 S02548 AMH S00111 ASPH S02425 BEAN1 S00215 C8ORF37 S00270 ABCA3 S00007 ADGRV1 S00058 AMHR2 S00112 ASPM S00159 BEST1 S00216 C9 S00271 ABCA4 S00008 ADIPOQ S00059 AMN S00113 ASS1 S00160 BFSP1 S02280 CA2 S00272 ABCA7 S02106 ADIPOR1 * S00060 AMPD1 S02670 ATAD3A * S02196 BFSP2 S00217 CA4 S02303 ABCB11 S00009 ADIPOR2 S00061 AMPD2 S02128 ATCAY S00162 BGN S02633 CA8 S00273 ABCB4 S00010 ADK S02595 AMT S00114 ATF6 S00163 BHLHA9 S00218 CABP2 S00274 ABCB6 S00011 ADNP S02320 ANG S00115 ATIC S02458 BICD2 S00220 CABP4 S00275 ABCB7 S00012 ADSL S00062 ANK1 S00116 ATL1 S00164 BIN1 S00221 CACNA1A S00276 ABCC2 S00013 AFF2 S00063 ANK2 S00117 ATL3 S00165 BLK S00222 CACNA1C * S00277 ABCC6 * S00014 AFG3L2 * S00064 ANKH S00118 ATM S00166 BLM S00223 CACNA1D S00278 ABCC8 S00015 AGA S00065 ANKRD11 * S02140 ATOH7 S02390 BLNK S02281 CACNA1F S00279 ABCC9 S00016 AGBL5 S02452 ANKS6 S00121 ATP13A2 S00168 BLOC1S3 S00224 CACNA1H S00280 ABCD1 * S00017 AGK * -

Expanded View Figures
Valentina Quarantotti et al The EMBO Journal Expanded View Figures Figure EV1. The effect of combined nocodazole A PCM1 locus and cytochalasin-B treatment on the distribution of endogenously labelled PCM1-GFP in DT40 cells. 32 33 3435 UTR A Diagram showing the GFP construct used to target the chicken PCM1 locus at the C-terminus on both alleles, by homologous recombination. Highlighted the Sal1 and BamH1 sites used for 2.8 kb 0.7 kb ~ 3 kb 3.3 kb restriction digestion to clone the LA (Left Arm) LA GFP Blasti/Puro/His RA and the RA (Right Arm) and to replace the resistance cassette. Clones were screened for Sal1BamH1 BamH1 Sal1 antibiotic resistance genes blasticidin (Blasti), puromycin (Puro) or Histidinol (His). LoxP sites B flanking the resistance cassette are represented by red triangles. The dashed lines indicate the sites of recombination and integration in the GFP γ-tubulin GFP, γ-tubulin, DNA PCM1 locus. Confirmation of targeting was carried out by Western blotting, as shown in Fig 1B–D. B Representative immunofluorescence images of DMSO cell lines with genotypes as indicated, treated with both nocodazole (2 lg/ml) and cytochalasin-B (1 lg/ml). DMSO-treated cells were used as a control (DMSO, upper panels). 2 PCM1-GFP Treatments were carried out for h, and cells were co-stained with antibodies against GFP WT (green) and c-tubulin (red). DNA is in blue. Nocodazole + Images correspond to maximum intensity Cytochalasin-B projections of confocal micrographs. Asterisks mark cells with dispersed satellites. Note that drug treatment leads to an increase in large and a decrease in small satellite granules in all three genotypes, but the effects are more prominent in acentriolar than in WT cells. -

Detection of H3k4me3 Identifies Neurohiv Signatures, Genomic
viruses Article Detection of H3K4me3 Identifies NeuroHIV Signatures, Genomic Effects of Methamphetamine and Addiction Pathways in Postmortem HIV+ Brain Specimens that Are Not Amenable to Transcriptome Analysis Liana Basova 1, Alexander Lindsey 1, Anne Marie McGovern 1, Ronald J. Ellis 2 and Maria Cecilia Garibaldi Marcondes 1,* 1 San Diego Biomedical Research Institute, San Diego, CA 92121, USA; [email protected] (L.B.); [email protected] (A.L.); [email protected] (A.M.M.) 2 Departments of Neurosciences and Psychiatry, University of California San Diego, San Diego, CA 92103, USA; [email protected] * Correspondence: [email protected] Abstract: Human postmortem specimens are extremely valuable resources for investigating trans- lational hypotheses. Tissue repositories collect clinically assessed specimens from people with and without HIV, including age, viral load, treatments, substance use patterns and cognitive functions. One challenge is the limited number of specimens suitable for transcriptional studies, mainly due to poor RNA quality resulting from long postmortem intervals. We hypothesized that epigenomic Citation: Basova, L.; Lindsey, A.; signatures would be more stable than RNA for assessing global changes associated with outcomes McGovern, A.M.; Ellis, R.J.; of interest. We found that H3K27Ac or RNA Polymerase (Pol) were not consistently detected by Marcondes, M.C.G. Detection of H3K4me3 Identifies NeuroHIV Chromatin Immunoprecipitation (ChIP), while the enhancer H3K4me3 histone modification was Signatures, Genomic Effects of abundant and stable up to the 72 h postmortem. We tested our ability to use H3K4me3 in human Methamphetamine and Addiction prefrontal cortex from HIV+ individuals meeting criteria for methamphetamine use disorder or not Pathways in Postmortem HIV+ Brain (Meth +/−) which exhibited poor RNA quality and were not suitable for transcriptional profiling. -

SUPPLEMENTARY MATERIALS and METHODS PBMC Transcriptomics
BMJ Publishing Group Limited (BMJ) disclaims all liability and responsibility arising from any reliance Supplemental material placed on this supplemental material which has been supplied by the author(s) Gut SUPPLEMENTARY MATERIALS AND METHODS PBMC transcriptomics identifies immune-metabolism disorder during the development of HBV-ACLF Contents l Supplementary methods l Supplementary Figure 1 l Supplementary Figure 2 l Supplementary Figure 3 l Supplementary Figure 4 l Supplementary Figure 5 l Supplementary Table 1 l Supplementary Table 2 l Supplementary Table 3 l Supplementary Table 4 l Supplementary Tables 5-14 l Supplementary Table 15 l Supplementary Table 16 l Supplementary Table 17 Li J, et al. Gut 2021;0:1–13. doi: 10.1136/gutjnl-2020-323395 BMJ Publishing Group Limited (BMJ) disclaims all liability and responsibility arising from any reliance Supplemental material placed on this supplemental material which has been supplied by the author(s) Gut SUPPLEMENTARY METHODS Test for HBV DNA The levels of HBV DNA were detected using real-time PCR with a COBAS® AmpliPrep/COBAS® TaqMan 48 System (Roche, Basel, Switzerland) and HBV Test v2.0. Criteria for diagnosing cirrhosis Pathology The gold standard for the diagnosis of cirrhosis is a liver biopsy obtained through a percutaneous or transjugular approach.1 Ultrasonography was performed 2-4 hours before biopsy. Liver biopsy specimens were obtained by experienced physicians. Percutaneous transthoracic puncture of the liver was performed according to the standard criteria. After biopsy, patients were monitored in the hospital with periodic analyses of haematocrit and other vital signs for 24 hours. Cirrhosis was diagnosed according to the globally agreed upon criteria.2 Cirrhosis is defined based on its pathological features under a microscope: (a) the presence of parenchymal nodules, (b) differences in liver cell size and appearance, (c) fragmentation of the biopsy specimen, (d) fibrous septa, and (d) an altered architecture and vascular relationships. -

ZNF384-Related Fusion Genes Define a Subgroup of Childhood B-Cell
Acute Lymphoblastic Leukemia SUPPLEMENTARY APPENDIX ZNF384 -related fusion genes define a subgroup of childhood B-cell precursor acute lymphoblastic leukemia with a characteristic immunotype Shinsuke Hirabayashi, 1,2 Kentaro Ohki, 1 Kazuhiko Nakabayashi, 3 Hitoshi Ichikawa, 4 Yukihide Momozawa, 5 Kohji Oka - mura, 6 Akinori Yaguchi, 1,7 Kazuki Terada, 1 Yuya Saito, 1,8 Ai Yoshimi, 1,9 Hiroko Ogata-Kawata, 3 Hiromi Sakamoto, 4 Moto - hiro Kato, 1,10 Junya Fujimura, 7 Moeko Hino, 11 Akitoshi Kinoshita, 12 Harumi Kakuda, 13 Hidemitsu Kurosawa, 14 Keisuke Kato, 9 Ryosuke Kajiwara, 15 Koichi Moriwaki, 16 Tsuyoshi Morimoto, 17 Kozue Nakamura, 18 Yasushi Noguchi, 19 Tomoo Osumi, 1,20 Kazuo Sakashita, 21 Junko Takita, 22 Yuki Yuza, 8 Koich Matsuda, 23 Teruhiko Yoshida, 4 Kenji Matsumoto, 24 Kenichiro Hata, 3 Michiaki Kubo, 5 Yoichi Matsubara, 25 Takashi Fukushima, 26 Katsuyoshi Koh, 27 Atsushi Manabe, 2 Akira Ohara 28 and Nobutaka Kiyokawa 1 for the Tokyo Children’s Cancer Study Group (TCCSG) 1Department of Pediatric Hematology and Oncology Research, National Research Institute for Child Health and Development, Seta - gaya-ku, Tokyo; 2Department of Pediatrics, St. Luke's International Hospital, Chuo-ku, Tokyo; 3Department of Maternal-Fetal Biology, National Research Institute for Child Health and Development, Setagaya-ku, Tokyo; 4Division of Genetics, National Cancer Center Re - search Institute, Chuo-ku, Tokyo; 5Laboratory for Genotyping Development, Center for Integrative Medical Sciences (IMS), RIKEN, Yoko - hama-shi, Kanagawa; 6Department -

Identifying Human Interactors of SARS-Cov-2 Proteins and Drug Targets for COVID-19 Using Network-Based Label Propagation Arxiv:2
Identifying Human Interactors of SARS-CoV-2 Proteins and Drug Targets for COVID-19 using Network-Based Label Propagation Jeffrey N. Law1, Kyle Akers1, Nure Tasnina2, Catherine M. Della Santina3, Meghana Kshirsagar4, Judith Klein-Seetharaman5, Mark Crovella6, Padmavathy Rajagopalan7, Simon Kasif3, and T. M. Murali2,* 1Interdisciplinary Ph.D. Program in Genetics, Bioinformatics, and Computational Biology, Blacksburg, VA, USA 2Department of Computer Science, Virginia Tech, Blacksburg, VA, USA 3Department of Biomedical Engineering, Boston University, Boston, MA, USA 4AI for Good Lab, Microsoft, Redmond, WA, USA 5Department of Chemistry, Colorado School of Mines, Golden, CO USA 6Department of Computer Science, Boston University, Boston, MA, USA 7Department of Chemical Engineering, Virginia Tech, Blacksburg, VA, USA *Corresponding author. Email: [email protected] Motivated by the critical need to identify new treatments for COVID- arXiv:2006.01968v2 [q-bio.MN] 22 Jun 2020 19, we present a genome-scale, systems-level computational approach to prioritize drug targets based on their potential to regulate host- virus interactions or their downstream signaling targets. We adapt and specialize network label propagation methods to this end. We demonstrate that these techniques can predict human-SARS-CoV- 2 protein interactors with high accuracy. The top-ranked proteins 1 that we identify are enriched in host biological processes that are po- tentially coopted by the virus. We present cases where our method- ology generates promising insights such as the potential role of HSPA5 in viral entry. We highlight the connection between en- doplasmic reticulum stress, HSPA5, and anti-clotting agents. We identify tubulin proteins involved in ciliary assembly that are tar- geted by anti-mitotic drugs.