Common Cutaneous Dermatophyte Infections of the Skin and Nails
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Fungal Infections from Human and Animal Contact
Journal of Patient-Centered Research and Reviews Volume 4 Issue 2 Article 4 4-25-2017 Fungal Infections From Human and Animal Contact Dennis J. Baumgardner Follow this and additional works at: https://aurora.org/jpcrr Part of the Bacterial Infections and Mycoses Commons, Infectious Disease Commons, and the Skin and Connective Tissue Diseases Commons Recommended Citation Baumgardner DJ. Fungal infections from human and animal contact. J Patient Cent Res Rev. 2017;4:78-89. doi: 10.17294/2330-0698.1418 Published quarterly by Midwest-based health system Advocate Aurora Health and indexed in PubMed Central, the Journal of Patient-Centered Research and Reviews (JPCRR) is an open access, peer-reviewed medical journal focused on disseminating scholarly works devoted to improving patient-centered care practices, health outcomes, and the patient experience. REVIEW Fungal Infections From Human and Animal Contact Dennis J. Baumgardner, MD Aurora University of Wisconsin Medical Group, Aurora Health Care, Milwaukee, WI; Department of Family Medicine and Community Health, University of Wisconsin School of Medicine and Public Health, Madison, WI; Center for Urban Population Health, Milwaukee, WI Abstract Fungal infections in humans resulting from human or animal contact are relatively uncommon, but they include a significant proportion of dermatophyte infections. Some of the most commonly encountered diseases of the integument are dermatomycoses. Human or animal contact may be the source of all types of tinea infections, occasional candidal infections, and some other types of superficial or deep fungal infections. This narrative review focuses on the epidemiology, clinical features, diagnosis and treatment of anthropophilic dermatophyte infections primarily found in North America. -

Introduction to Mycology
INTRODUCTION TO MYCOLOGY The term "mycology" is derived from Greek word "mykes" meaning mushroom. Therefore mycology is the study of fungi. The ability of fungi to invade plant and animal tissue was observed in early 19th century but the first documented animal infection by any fungus was made by Bassi, who in 1835 studied the muscardine disease of silkworm and proved the that the infection was caused by a fungus Beauveria bassiana. In 1910 Raymond Sabouraud published his book Les Teignes, which was a comprehensive study of dermatophytic fungi. He is also regarded as father of medical mycology. Importance of fungi: Fungi inhabit almost every niche in the environment and humans are exposed to these organisms in various fields of life. Beneficial Effects of Fungi: 1. Decomposition - nutrient and carbon recycling. 2. Biosynthetic factories. The fermentation property is used for the industrial production of alcohols, fats, citric, oxalic and gluconic acids. 3. Important sources of antibiotics, such as Penicillin. 4. Model organisms for biochemical and genetic studies. Eg: Neurospora crassa 5. Saccharomyces cerviciae is extensively used in recombinant DNA technology, which includes the Hepatitis B Vaccine. 6. Some fungi are edible (mushrooms). 7. Yeasts provide nutritional supplements such as vitamins and cofactors. 8. Penicillium is used to flavour Roquefort and Camembert cheeses. 9. Ergot produced by Claviceps purpurea contains medically important alkaloids that help in inducing uterine contractions, controlling bleeding and treating migraine. 10. Fungi (Leptolegnia caudate and Aphanomyces laevis) are used to trap mosquito larvae in paddy fields and thus help in malaria control. Harmful Effects of Fungi: 1. -

Molecular Analysis of Dermatophytes Suggests Spread of Infection Among Household Members
Molecular Analysis of Dermatophytes Suggests Spread of Infection Among Household Members Mahmoud A. Ghannoum, PhD; Pranab K. Mukherjee, PhD; Erin M. Warshaw, MD; Scott Evans, PhD; Neil J. Korman, MD, PhD; Amir Tavakkol, PhD, DipBact Practice Points When a patient presents with tinea pedis or onychomycosis, inquire if other household members also have the infection, investigate if they have a history of concomitant tinea pedis and onychomycosis, and examine for plantar scaling and/or nail discoloration. If the variables above areCUTIS observed, think about spread of infection and treatment options. Dermatophyte infection from the same strains may Drs. Ghannoum, Mukherjee, and Korman are from University be an important route for transmission of derma- Hospitals Case Medical Center, Cleveland, Ohio. Dr. Warshaw is from the University of Minnesota, Minneapolis, and Minneapolis Veterans tophytoses within a household. In this study, we AffairsDo Medical Center. Dr. Evans is from Notthe Harvard School of Public used molecularCopy methods to identify dermatophytes Health, Boston, Massachusetts. Dr. Tavakkol was from Novartis in members of dermatophyte-infected households Pharmaceuticals Corporation, East Hanover, New Jersey, and and evaluated variables associated with the currently is from Topica Pharmaceuticals, Inc, Los Altos, California. spread of infection. Fungal species were identi- This article was supported by a grant from Novartis Pharmaceuticals Corporation. Dr. Ghannoum has served as a consultant and/or fied by polymerase chain reaction (PCR) using speaker for and has received grants and contracts from Merck & Co, primers targeting the internal transcribed spacer Inc; Novartis Pharmaceuticals Corporation; Pfizer Inc; and Stiefel, (ITS) regions (ITS1 and ITS4). For strain differen- a GSK company. -

Dermatophyte and Non-Dermatophyte Onychomycosis in Singapore
Australas J. Dermatol 1992; 33: 159-163 DERMATOPHYTE AND NON-DERMATOPHYTE ONYCHOMYCOSIS IN SINGAPORE JOYCE TENG-EE LIM, HOCK CHENG CHUA AND CHEE LEOK GOH Singapore SUMMARY Onychomycosis is caused by dermatophytes, moulds and yeasts. It is important to identify the non-dermatophyte moulds as they are resistant to the usual anti-fungals. A prospective study was undertaken in the National Skin Centre, Singapore to study the pattern of dermatophyte and non-dermatophyte onychomycosis. 53 male and 47 female patients seen between June 1990 and June 1991 were entered into the study. Direct microscopy was done and the nail clippings were cultured. Toe and finger nails were equally infected. Dermatophytes were isolated from 21 patients namely, T. rubrum (12/21), T. interdigitale (5/21), T. mentagrophytes (3/21) and T. violaceum (1/21). Candida onychomycosis occurred in 39 patients and was caused by C. albicans (38/39) and C. parapsilosis (1/39). 37/39 patients had associated paronychia. 5 types of moulds were isolated from 12 patients, namely Fusarium species (6/12), Aspergillus species (3/12), S. brevicaulis (1/12), Aureobasilium species (1/12) and Penicillium species (1/12). Although the clinical pattern and microscopy may predict the type of organisms, in practice this is difficult. Only cultures were confirmatory. 28% (28/100) had negative cultures despite a positive microscopy, and moulds (12/100) grown might be contaminants rather than pathogens. Key words: Moulds, yeasts, fungi, tinea, onychomycosis, dermatophyte, non-dermatophyte INTRODUCTION METHODS AND MATERIALS Onychomycosis, a common nail disorder, is 100 consecutive patients, seen in our centre caused by dermatophytes, non-dermatophyte between June 1990 and June 1991, with a new moulds, or yeasts. -

Acquired Immunodeficiency Syndrome (AIDS), 1-23 Aspergillosis In, 16-18
Index Acquired immunodeficiency syndrome enzyme activities and, 336-339 (AIDS), 1-23 fatty acid synthesis and, 334-336 aspergillosis in, 16-18 ergosterol synthesis inhibition by, candidiasis in, 6-11 316-320 coccidioidomycosis in, 15--16 membrane and cell functions and 14 cryptococcosis in, 11-15 alpha-methylsterol effects, 326- histoplasmosis in, 15--16 329 host resistance in, 6 membrane lipid interactions of, 339- immunofluorescent staining for tissue 341 section in, 4-5 selective toxicity of, 325--326 Malassezia furfur in, 21 Antigen nocardiosis in, 18-20 of Aspergillus fumigatus, 198-199 pathologic diagnosis in, 3-4 advancing line immunoelectrophoresis Actinomyces, monoclonal antibodies in, for, 176 196 of Blastomyces dermatitidis, 199 Adriamycin, 133 of Candida albicans, 199-200 Adrucil, 133 of Coccidioides immitis, 200 Aerosporin, 114 of Cryptococcus neoformans, 200--201 Alkeran, 133 crossed immunoelectrophoresis for, Amantadine HCI, 140 174-175 Amikacin sulfate, 107 crossed-tandon immunoelectrophoresis Amikin,107 for, 176-178 Aminoglycoside, 106f, 107 of dermatophyte, 201 Aminosalicylic acid, 123-124 of Histoplasma capsulatum, 201-202 Amphotericin B, 127 methods for determining chemical na- Ancobon, 127-128 ture of, 180 Antifungal agents, systemic, 126f, 127- monitoring isolation of, 176-181 129 rocket immunoelectrophoresis for, 176 Antifungal azole derivatives, 313-351 of Sporothrix schenckii, 202 cytochrome P-450 interation with, of zygomycetes, 202 320--323 Antineoplastic agents, 131-135, 132f,134t ergosterol depletion effects -

Managing Athlete's Foot
South African Family Practice 2018; 60(5):37-41 S Afr Fam Pract Open Access article distributed under the terms of the ISSN 2078-6190 EISSN 2078-6204 Creative Commons License [CC BY-NC-ND 4.0] © 2018 The Author(s) http://creativecommons.org/licenses/by-nc-nd/4.0 REVIEW Managing athlete’s foot Nkatoko Freddy Makola,1 Nicholus Malesela Magongwa,1 Boikgantsho Matsaung,1 Gustav Schellack,2 Natalie Schellack3 1 Academic interns, School of Pharmacy, Sefako Makgatho Health Sciences University 2 Clinical research professional, pharmaceutical industry 3 Professor, School of Pharmacy, Sefako Makgatho Health Sciences University *Corresponding author, email: [email protected] Abstract This article is aimed at providing a succinct overview of the condition tinea pedis, commonly referred to as athlete’s foot. Tinea pedis is a very common fungal infection that affects a significantly large number of people globally. The presentation of tinea pedis can vary based on the different clinical forms of the condition. The symptoms of tinea pedis may range from asymptomatic, to mild- to-severe forms of pain, itchiness, difficulty walking and other debilitating symptoms. There is a range of precautionary measures available to prevent infection, and both oral and topical drugs can be used for treating tinea pedis. This article briefly highlights what athlete’s foot is, the different causes and how they present, the prevalence of the condition, the variety of diagnostic methods available, and the pharmacological and non-pharmacological management of the -

P2399 Lateral Flow Immunoassay for Rapid Detection of Dermatophytes in Clinical Specimens
P2399 Lateral flow immunoassay for rapid detection of dermatophytes in clinical specimens Amanda Burnham-Marusich*1, Caitlyn Orne1 2, Alexander Kvam3, Heather Green2, Alexandra Myers4, Aline Rodrigues Hoffmann4, Amy Crum5, Mahmoud Ghannoun6, Thomas Kozel1 3 1DxDiscovery, Reno, United States, 2University of Nevada, Reno, Reno, United States, 3University of Nevada, Reno School of Medicine, Reno, United States, 4Texas A&M University, College Station, United States, 5Houston SPCA, Houston, United States, 6Case Western Reserve University, Cleveland, United States Background: Fungal skin infections affect approximately 1 billion people each year. Most infections are treated with topical antifungal agents. Other infections, e.g., tinea capitis and onychomycosis, require use of oral antifungal agents that may produce significant side effects in some patients. Laboratory confirmation of fungal infection prior to use of antifungal agents is recommended but often not done due, in part, to the time and cost of laboratory testing. The goal of this study was to develop a lateral flow immunoassay (LFIA) for rapid diagnosis of dermatophytosis. Materials/methods: Monoclonal antibody (mAb) 2DA6 was produced from splenocytes of mice immunized with fungal cell wall fragments. Hybridomas were generated using standard methods. Results: A LFIA was constructed from mAb 2DA6 for detection of mannans of dermatophyte fungi in clinical samples. The antibody is reactive with the alpha-1,6 mannose backbone in mannans of fungi of the Zygomycota and the Ascomycota. However, mAb 2DA6 has an exquisite sensitivity for mannans of dermatophytes and the Zygomycota due to an apparent low level of side chain substitution found on these mannans vs. high levels of side chain substitution that occludes the backbone in mannans of other fungi. -
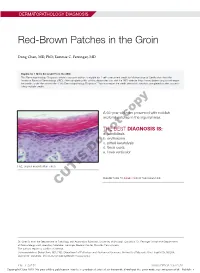
Red-Brown Patches in the Groin
DERMATOPATHOLOGY DIAGNOSIS Red-Brown Patches in the Groin Dong Chen, MD, PhD; Tammie C. Ferringer, MD Eligible for 1 MOC SA Credit From the ABD This Dermatopathology Diagnosis article in our print edition is eligible for 1 self-assessment credit for Maintenance of Certification from the American Board of Dermatology (ABD). After completing this activity, diplomates can visit the ABD website (http://www.abderm.org) to self-report the credits under the activity title “Cutis Dermatopathology Diagnosis.” You may report the credit after each activity is completed or after accumu- lating multiple credits. A 66-year-old man presented with reddish arciform patchescopy in the inguinal area. THE BEST DIAGNOSIS IS: a. candidiasis b. noterythrasma c. pitted keratolysis d. tinea cruris Doe. tinea versicolor H&E, original magnification ×600. PLEASE TURN TO PAGE 419 FOR THE DIAGNOSIS CUTIS Dr. Chen is from the Department of Pathology and Anatomical Sciences, University of Missouri, Columbia. Dr. Ferringer is from the Departments of Dermatology and Laboratory Medicine, Geisinger Medical Center, Danville, Pennsylvania. The authors report no conflict of interest. Correspondence: Dong Chen, MD, PhD, Department of Pathology and Anatomical Sciences, University of Missouri, One Hospital Dr, MA204, DC018.00, Columbia, MO 65212 ([email protected]). 416 I CUTIS® WWW.MDEDGE.COM/CUTIS Copyright Cutis 2018. No part of this publication may be reproduced, stored, or transmitted without the prior written permission of the Publisher. DERMATOPATHOLOGY DIAGNOSIS DISCUSSION THE DIAGNOSIS: Erythrasma rythrasma usually involves intertriginous areas surface (Figure 1) compared to dermatophyte hyphae that (eg, axillae, groin, inframammary area). Patients tend to be parallel to the surface.2 E present with well-demarcated, minimally scaly, red- Pitted keratolysis is a superficial bacterial infection brown patches. -

Tinea Capitis 2014 L.C
BJD GUIDELINES British Journal of Dermatology British Association of Dermatologists’ guidelines for the management of tinea capitis 2014 L.C. Fuller,1 R.C. Barton,2 M.F. Mohd Mustapa,3 L.E. Proudfoot,4 S.P. Punjabi5 and E.M. Higgins6 1Department of Dermatology, Chelsea & Westminster Hospital, Fulham Road, London SW10 9NH, U.K. 2Department of Microbiology, Leeds General Infirmary, Leeds LS1 3EX, U.K. 3British Association of Dermatologists, Willan House, 4 Fitzroy Square, London W1T 5HQ, U.K. 4St John’s Institute of Dermatology, Guy’s and St Thomas’ NHS Foundation Trust, St Thomas’ Hospital, Westminster Bridge Road, London SE1 7EH, U.K. 5Department of Dermatology, Hammersmith Hospital, 150 Du Cane Road, London W12 0HS, U.K. 6Department of Dermatology, King’s College Hospital, Denmark Hill, London SE5 9RS, U.K. 1.0 Purpose and scope Correspondence Claire Fuller. The overall objective of this guideline is to provide up-to- E-mail: [email protected] date, evidence-based recommendations for the management of tinea capitis. This document aims to update and expand Accepted for publication on the previous guidelines by (i) offering an appraisal of 8 June 2014 all relevant literature since January 1999, focusing on any key developments; (ii) addressing important, practical clini- Funding sources cal questions relating to the primary guideline objective, i.e. None. accurate diagnosis and identification of cases; suitable treat- ment to minimize duration of disease, discomfort and scar- Conflicts of interest ring; and limiting spread among other members of the L.C.F. has received sponsorship to attend conferences from Almirall, Janssen and LEO Pharma (nonspecific); has acted as a consultant for Alliance Pharma (nonspe- community; (iii) providing guideline recommendations and, cific); and has legal representation for L’Oreal U.K. -

25 Chrysosporium
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Universidade do Minho: RepositoriUM 25 Chrysosporium Dongyou Liu and R.R.M. Paterson contents 25.1 Introduction ..................................................................................................................................................................... 197 25.1.1 Classification and Morphology ............................................................................................................................ 197 25.1.2 Clinical Features .................................................................................................................................................. 198 25.1.3 Diagnosis ............................................................................................................................................................. 199 25.2 Methods ........................................................................................................................................................................... 199 25.2.1 Sample Preparation .............................................................................................................................................. 199 25.2.2 Detection Procedures ........................................................................................................................................... 199 25.3 Conclusion .......................................................................................................................................................................200 -

Prevalence & Distribution of Keratinophilic Fungi in Relation To
African Journal of Microbiology Research Vol. 6(42), pp. 6973-6977, 6 November, 2012 Available online at http://www.academicjournals.org/AJMR DOI: 10.5897/AJMR12.897 ISSN 1996-0808 ©2012 Academic Journals Full Length Research Paper A descriptive study of keratinophilic fungal flora of animal and bird habitat, Jaipur, Rajasthan Neetu Jain* and Meenakshi Sharma Laboratory of Microbiology, Department of Botany, University of Rajasthan, Jaipur India. Accepted 30 May, 2012 Keratinophilic fungi occur abundantly in the superficial soil layer of landfills and their surrounding. Forty seven soil samples of animal (37 samples) and bird (10 samples) habitats from different localities of Jaipur District were collected for the estimation of keratinophilic fungi using the hair baiting technique. Seventy five isolates belonging to 14 genera and 20 species were reported. Soil pH range varies from 6.5 to 10.5. But most of the fungi (33.33%) were isolated from neutral soil (pH 7.0). Chrysosporium tropicum (25.33%) was the predominant fungi isolated from both habitats soil. This was followed by the predominance of Trichophyton terrestre (12%), Trichophyton mentagrophytes (9.3%), C. indicum (5.33%), Actinomyces sp. (6.67%), and Nocardia sp. (6.67%) in both habitats. Interestingly, Exserophilum sp., Microsporum audouinii, Trichophyton verrucosum were isolated for the first time from Jaipur India. Key words: Dermatophytes, Trichophyton, Microsporum, Chrysosporium, soil fungi. INTRODUCTION Keratinophilic fungi include a variety of filamentous fungi may be exploited for their biotechnological potential in mainly comprising of hyphomycetes and several other industry (Kaul and Sumbali, 1999). Keratinophilic fungi taxonomic groups. Hypomycetes include dermatophytes are generally considered as soil saprophytes (Ajello, and a great variety of non dermatophytic filamentous 1953). -

Detection of Histoplasma DNA from Tissue Blocks by a Specific
Journal of Fungi Article Detection of Histoplasma DNA from Tissue Blocks by a Specific and a Broad-Range Real-Time PCR: Tools to Elucidate the Epidemiology of Histoplasmosis Dunja Wilmes 1,*, Ilka McCormick-Smith 1, Charlotte Lempp 2 , Ursula Mayer 2 , Arik Bernard Schulze 3 , Dirk Theegarten 4, Sylvia Hartmann 5 and Volker Rickerts 1 1 Reference Laboratory for Cryptococcosis and Uncommon Invasive Fungal Infections, Division for Mycotic and Parasitic Agents and Mycobacteria, Robert Koch Institute, 13353 Berlin, Germany; [email protected] (I.M.-S.); [email protected] (V.R.) 2 Vet Med Labor GmbH, Division of IDEXX Laboratories, 71636 Ludwigsburg, Germany; [email protected] (C.L.); [email protected] (U.M.) 3 Department of Medicine A, Hematology, Oncology and Pulmonary Medicine, University Hospital Muenster, 48149 Muenster, Germany; [email protected] 4 Institute of Pathology, University Hospital Essen, University Duisburg-Essen, 45147 Essen, Germany; [email protected] 5 Senckenberg Institute for Pathology, Johann Wolfgang Goethe University Frankfurt, 60323 Frankfurt am Main, Germany; [email protected] * Correspondence: [email protected]; Tel.: +49-30-187-542-862 Received: 10 November 2020; Accepted: 25 November 2020; Published: 27 November 2020 Abstract: Lack of sensitive diagnostic tests impairs the understanding of the epidemiology of histoplasmosis, a disease whose burden is estimated to be largely underrated. Broad-range PCRs have been applied to identify fungal agents from pathology blocks, but sensitivity is variable. In this study, we compared the results of a specific Histoplasma qPCR (H. qPCR) with the results of a broad-range qPCR (28S qPCR) on formalin-fixed, paraffin-embedded (FFPE) tissue specimens from patients with proven fungal infections (n = 67), histologically suggestive of histoplasmosis (n = 36) and other mycoses (n = 31).