Magnetic Stimulation of the Central and Peripheral Nervous Systems
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
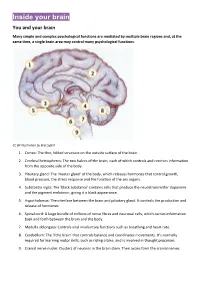
Inside Your Brain You and Your Brain
Inside your brain You and your brain Many simple and complex psychological functions are mediated by multiple brain regions and, at the same time, a single brain area may control many psychological functions. CC BY Illustration by Bret Syfert 1. Cortex: The thin, folded structure on the outside surface of the brain. 2. Cerebral hemispheres: The two halves of the brain, each of which controls and receives information from the opposite side of the body. 3. Pituitary gland: The ‘master gland’ of the body, which releases hormones that control growth, blood pressure, the stress response and the function of the sex organs. 4. Substantia nigra: The ‘black substance’ contains cells that produce the neurotransmitter dopamine and the pigment melatonin, giving it a black appearance. 5. Hypothalamus: The interface between the brain and pituitary gland. It controls the production and release of hormones. 6. Spinal cord: A large bundle of millions of nerve fibres and neuronal cells, which carries information back and forth between the brain and the body. 7. Medulla oblongata: Controls vital involuntary functions such as breathing and heart rate. 8. Cerebellum: The ‘little brain’ that controls balance and coordinates movements. It’s normally required for learning motor skills, such as riding a bike, and is involved in thought processes. 9. Cranial nerve nuclei: Clusters of neurons in the brain stem. Their axons form the cranial nerves. Your brain underpins who you are. It stores your knowledge and memories, gives you the capacity for thought and emotion, and enables you to control your body. The brain is just one part of the nervous system. -

Clinical Neurophysiology Board Review Q&A
Clinical Neurophysiology Board Review Board Clinical Neurophysiology Clinical Neurophysiology Board Review Q&A Clinical Puneet K. Gupta, MD, MSE • Pradeep N. Modur, MD, MS • Srikanth Muppidi, MD his high-yield, illustrated clinical neurophysiology board review is a comprehen- Neurophysiology sive resource for assessing and refining the knowledge tested on multiple board Texaminations. Written by authors who are collectively board certified in all of the areas covered, the book is a valuable study tool for candidates preparing for certifica- tion or recertification in clinical neurophysiology, neuromuscular medicine, epilepsy, Board Review sleep medicine, and neurology. Using structured question formats typically encountered on boards, this comprehensive review allows users to assess their knowledge in a wide range of topics, provides rationales for correct answers, and explains why the other choices are incorrect. A unique “Pearls” section at the end of the book allows for quick review of the most important concepts prior to exam day. Clinical Neurophysiology Board Review Q&A contains 801 questions with answers and detailed explanations. The book is divided into eight chapters covering anatomy Q and physiology, electronics and instrumentation, nerve conduction studies and EMG, & EEG, evoked potentials and intraoperative monitoring, sleep studies, ethics and safety, and advanced topics including QEEG, MEG, TES, autonomic testing, and more. A Liberal use of image-based questions illustrating the full spectrum of neurophysiologic & tests and findings build interpretive skills. Questions are randomized and include Q A both case-related questions in series and stand-alone items to familiarize candidates Gu with the question types and formats they will find on the exam. -

The Peripheral Nerves: Update on Ultrasound and Magnetic Resonance Imaging I
The peripheral nerves: update on ultrasound and magnetic resonance imaging I. Möller1, M. Miguel2, D.A. Bong1, F. Zaottini3, C. Martinoli4 1Instituto Poal de Reumatologia, ABSTRACT of the nerve along its trajectory along University of Barcelona, Spain, The motor and sensory branches of with immediate one-to-one compari- and EULAR Working Group Anatomy the somatic peripheral nervous system son with the contralateral structures (6, for the Image; (PNS) can be visualised by different im- 7). In addition, US-guidance has led to 2Department of Pathology and Experimental Therapeutics, Human aging systems. This article focuses on the development of a variety of inter- Anatomy and Embryology Unit, imaging of peripheral nerves by mag- ventional procedures. The use of US is University of Barcelona, Spain; netic resonance imaging (MRI) and becoming widespread in providing ac- 3Department of Health Sciences, high-resolution ultrasound (US). The curate and safe regional anesthesia as DISSAL, University of Genoa; anatomic basis of the peripheral nerve well as focal and regional pain manage- 4 Department of Health Science, image, common pathologies and clini- ment. It has also becoming an increas- University of Genoa, Ospedale cal value of US and MRI imaging of pe- ingly important component of muscu- Policlinico San Martino, Genoa, Italy. ripheral nerves are reviewed. loskeletal specialties such as physical Ingrid Möller, MD medicine and rehabilitation and sports Maribel Miguel, MD David A. Bong, MD Introduction medicine. Federico Zaottini, MD Nerve pathology may be a cause of Carlo Martinoli, MD chronic pain and disability. The initial Anatomical considerations Please address correspondence to: diagnostic evaluation of the periph- The PNS includes spinal nerves that Dr David A. -

Supporting Individuals with Grief: Using a Psychological First Aid Framework Barney Dunn, July 2020 My Background and Acknowledgements
Supporting individuals with grief: Using a psychological first aid framework Barney Dunn, July 2020 My background and acknowledgements • Professor of Clinical Psychology at Mood Disorders Centre, University of Exeter and co-lead AccEPT clinic (NHS ‘post IAPT gap’ service) • Main area depression but been involved in developing some grief guidance as part of COVID response with Kathy Shear (Columbia) and Anke Ehlers (Oxford) • These materials developed to support counsellors/therapists to work with grief with principals of psychological first aid. • Thanks to Kathy Shear for letting me use some of her centre’s materials and videos Overview • Part 1: Understanding grief • Part 2: Supporting individuals through acute grief • Part 3: Use of psychological first aid framework for grief (and more generally) • Part 4: Prolonged grief disorder Learning objectives • Be familiar with range of grief reactions • Be able use your common factor skills and knowledge around bereavement from workshop to respond empathically and supportively to people who are grieving in a non-pathologizing way • Be able to use the psychological first aid framework to help people look after themselves while grieving • To know who and how to signpost on to further help • You are not expected to be experts in grief or to ‘treat’ grief Optional core reading Supporting people with grief: • Shear, M. K., Muldberg, S., & Periyakoil, V. (2017). Supporting patients who are bereaved. BMJ (Clinical research ed.), 358, j2854. https://doi.org/10.1136/bmj.j2854 • See also material for clinicians and public on this website: https://complicatedgrief.columbia.edu/professionals/complicated-grief-professionals/overview/ Prolonged grief disorder: • Jordan, A. -

EMG (Electromyography) And/Or NCS (Nerve Conduction Studies)
Tempe ñ Phoenix ñ Gilbert ñ Scottsdale ñ Peoria ñ Show Low PHONE: (480) 962-0071 www.SonoranSpine.com ñ www.SpineResearch.org Patient Name: Date: Date of Birth: S O N O R A N S P I N E -- R E F E R E N C E Electromyography and Nerve Conduction Studies An electromyogram (EMG) measures the electrical The electrode will be moved a number of times to activity of muscles at rest and during contraction. record the activity in different areas of the muscle or in Nerve conduction studies (NCS) measure how well and different muscles. how fast the nerves can send electrical signals. Nerve conduction studies If you have leg pain or numbness, you may undergo Several electrodes are attached to your skin. Several these tests to determine how much your nerves are quick electrical pulses are given to the nerve, and the being affected. These tests check how well the spinal time it takes for the muscle to contract in response to nerves and the nerves in your arms and legs are the electrical pulse is recorded. The speed of the working and whether there is nerve irritation or response is called the conduction velocity. The same damage. They do not test for pain. nerves on the other side of the body may be studied for comparison. When the test is done, the electrodes Why It Is Done are removed. An EMG is done to determine muscle tissue or nerve damage. It can find the cause of weakness, paralysis, How It Feels or muscle twitching. -

Microglia and Macrophages in the Pathological Central and Peripheral Nervous Systems
cells Review Microglia and Macrophages in the Pathological Central and Peripheral Nervous Systems Naoki Abe 1, Tasuku Nishihara 1,*, Toshihiro Yorozuya 1 and Junya Tanaka 2 1 Department of Anesthesia and Perioperative Medicine, Ehime University Graduate School of Medicine, Toon, Ehime 791-0295, Japan; [email protected] (N.A.); [email protected] (T.Y.) 2 Department of Molecular and cellular Physiology, Ehime University Graduate School of Medicine, Toon, Ehime 791-0295, Japan; [email protected] * Correspondence: [email protected]; Tel.: +81-89-960-5383; Fax: +81-89-960-5386 Received: 4 August 2020; Accepted: 17 September 2020; Published: 21 September 2020 Abstract: Microglia, the immunocompetent cells in the central nervous system (CNS), have long been studied as pathologically deteriorating players in various CNS diseases. However, microglia exert ameliorating neuroprotective effects, which prompted us to reconsider their roles in CNS and peripheral nervous system (PNS) pathophysiology. Moreover, recent findings showed that microglia play critical roles even in the healthy CNS. The microglial functions that normally contribute to the maintenance of homeostasis in the CNS are modified by other cells, such as astrocytes and infiltrated myeloid cells; thus, the microglial actions on neurons are extremely complex. For a deeper understanding of the pathophysiology of various diseases, including those of the PNS, it is important to understand microglial functioning. In this review, we discuss both the favorable and unfavorable roles of microglia in neuronal survival in various CNS and PNS disorders. We also discuss the roles of blood-borne macrophages in the pathogenesis of CNS and PNS injuries because they cooperatively modify the pathological processes of resident microglia. -

The Neural Basis of the Sense of Fatigue
Iowa State University Capstones, Theses and Graduate Theses and Dissertations Dissertations 2020 The neural basis of the sense of fatigue Mark Edward Hartman Iowa State University Follow this and additional works at: https://lib.dr.iastate.edu/etd Recommended Citation Hartman, Mark Edward, "The neural basis of the sense of fatigue" (2020). Graduate Theses and Dissertations. 18136. https://lib.dr.iastate.edu/etd/18136 This Dissertation is brought to you for free and open access by the Iowa State University Capstones, Theses and Dissertations at Iowa State University Digital Repository. It has been accepted for inclusion in Graduate Theses and Dissertations by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. The neural basis of the sense of fatigue by Mark Edward Hartman A dissertation submitted to the graduate faculty in partial fulfillment of the requirements for the degree of DOCTOR OF PHILOSOPHY Major: Kinesiology Program of Study Committee: Panteleimon Ekkekakis, Major Professor Peter Clark James Lang Jacob Meyer Rick Sharp The student author, whose presentation of the scholarship herein was approved by the program of study committee, is solely responsible for the content of this dissertation. The Graduate College will ensure this dissertation is globally accessible and will not permit alterations after a degree is conferred. Iowa State University Ames, Iowa 2020 Copyright © Mark Edward Hartman, 2020. All rights reserved. ii DEDICATION This dissertation is dedicated to my mother. Thank you for all your incredible support, encouragement, and patience, especially considering that it has taken me 14 years to finish my post-secondary education. -

Shame Handout for Dell Childrens
Fall 08 Dell Children's Medical Center January 2015 Shame, Relevant Neurobiology, and Treatment Implications Arlene Montgomery Ph.D., LCSW Shame Guilt Early-forming (before age two) Must have concept of another (2 yrs+) Implicit memory (amygdala) Explicit memory (upper right & left hemi) Arousal involves entire body: Arousal is an upper cortex, primarily left, • excitement state of worry • painful arousal (SNS) • counter-regulate (PNS) • positive outcome = repair • negative outcome = no repair; lingering in PNS state Non-narrative (not conscious) Narrative (conscious experience) • a state of being: "I am something..." • "I did something" e.g., bad, worthless, flawed as a whole • an act person Runs away from further external Seeks to maintain attachments interactions, but cannot run away from the internal representations of the scorn of the other - an internal experience Shame states may engage the freeze Guilt experiences may engage the more (dissociated, passive) state or flight activated fight states ( not actually fighting, state(hiding, other avoidant behaviors) or but actively making thing right, correcting submit state(agreeing, becoming invisible, mistakes, engaging the milieu in some way complying) to manage the painful arousal to address the “bad act, moving toward…) Repaired shame is a normative socialization experience (Schore, 2003 a,b) Relational trauma may result from unrepaired shame experiences "corroded connections", (B. Brown, 2010); "affecting global sense of self" (Lewis, 1971) Issues and Diagnoses PTSD: shame is predictor -

Nerve Conduction Studies: Essentials and Pitfalls in Practice
NERVE CONDUCTION STUDIES: J Neurol Neurosurg Psychiatry: first published as 10.1136/jnnp.2005.069138 on 16 June 2005. Downloaded from ESSENTIALS AND PITFALLS IN PRACTICE A Mallik, A I Weir ii23 J Neurol Neurosurg Psychiatry 2005;76(Suppl II):ii23–ii31. doi: 10.1136/jnnp.2005.069138 neurologist has sent a patient for nerve conduction studies (NCS) and has received the report, but what does it mean? We hope to remove some of the mysteries that may Asurround NCS. The techniques and how they are affected by disease are described in general terms. These principles can be applied to specific conditions discussed elsewhere. We also discuss the numerous pitfalls that may be encountered with NCS. Understanding these basic concepts will allow you to get the most from your clinical neurophysiology department. NCS are only part of a complete peripheral neurophysiological examination (PNE) and are frequently accompanied by a needle electromyogram (EMG). The combination of both techniques and those detailed in other articles in this issue are often required for a complete diagnostic study. The process of choosing the appropriate tests is the responsibility of the clinical neurophysiologist (CN) and not the referring doctor and is planned as a dynamic series of steps designed to answer specific questions about nervous system function raised by the clinical picture. c ABBREVIATIONS Clinical neurophysiologists can employ a confusing number of terms and abbreviations. Box 1 lists the ones we use frequently. WHAT DO YOU TELL THE PATIENT ABOUT THE TESTS? NCS involve activating nerves electrically with small safe pulses over several points on the skin of copyright. -

Patient Education Electromyogram (EMG) Nerve Conduction Study
Patient Education Electromyogram (EMG) Nerve Conduction Study Explanation of Test Your doctor has ordered a test called an EMG. EMG stands for electromyogram which loosely translated means electrical testing of muscles, but it has come to mean electrical testing of nerves and muscles. A specialist, the electromyographer, who has specialized training in the field of electromyography, performs the EMG. EMG is an in-office procedure that does not require hospitalization. On average, an EMG takes anywhere between 30 minutes and 2 hours, depending on how extensive your test is ordered to be. It can be done at any time during the day and, with few exceptions, does not require any special preparation. Sometimes EMGs are thought to be a treatment of some sort, or a type of acupuncture. This is not true. An EMG is only a test, much like an EKG or an x-ray. EMGs are usually ordered when you are having problems with your muscles or nerves. EMG’s test the muscles and nerves of your arms and legs to identify problems. Weakness of your muscles or “fatigue” (tiredness) may indicate nerve or muscle disease and require an EMG. Usually combined with nerve conduction studies (NCS), EMG’s are the most important diagnostic tests for the evaluation of neuropathy and myopathy (nerve and muscle disease). These tests are performed on motor and sensory nerves, but only large myelinated fibers can be evaluated by nerve conduction studies. EMG’s help diagnose conditions such as in carpal tunnel syndrome, polyneuropathy (as seen with diabetes), nutritional deficiencies, autoimmune processes, or degradation of the myelin (a type of coating around the nerve axon which allows the electrical signal to travel faster), as seen in Guillan Barre´ Syndrome. -
![Repetitive Transcranial Magnetic Stimulation [Rtms] for Panic Disorder](https://docslib.b-cdn.net/cover/3523/repetitive-transcranial-magnetic-stimulation-rtms-for-panic-disorder-1003523.webp)
Repetitive Transcranial Magnetic Stimulation [Rtms] for Panic Disorder
Repetitive transcranial magnetic stimulation (rTMS) for panic disorder in adults (Review) Li H, Wang J, Li C, Xiao Z This is a reprint of a Cochrane review, prepared and maintained by The Cochrane Collaboration and published in The Cochrane Library 2014, Issue 9 http://www.thecochranelibrary.com Repetitive transcranial magnetic stimulation (rTMS) for panic disorder in adults (Review) Copyright © 2014 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd. TABLE OF CONTENTS HEADER....................................... 1 ABSTRACT ...................................... 1 PLAINLANGUAGESUMMARY . 2 SUMMARY OF FINDINGS FOR THE MAIN COMPARISON . ..... 4 BACKGROUND .................................... 6 OBJECTIVES ..................................... 7 METHODS...................................... 7 Figure1. ..................................... 9 RESULTS....................................... 12 Figure2. ..................................... 13 Figure3. ..................................... 15 Figure4. ..................................... 16 DISCUSSION ..................................... 18 AUTHORS’CONCLUSIONS . 20 ACKNOWLEDGEMENTS . 21 REFERENCES..................................... 21 CHARACTERISTICSOFSTUDIES . .. 25 DATAANDANALYSES. 31 Analysis 1.3. Comparison 1 rTMS + pharmacotherapy versus sham rTMS + pharmacotherapy, Outcome 3 Acceptability: 1.dropoutsforanyreason. .33 Analysis 1.4. Comparison 1 rTMS + pharmacotherapy versus sham rTMS + pharmacotherapy, Outcome 4 Acceptability: 2.dropoutsforadverseeffects. .... 34 Analysis -

Grief Support Groups: Preference for Online Vs
Fort Hays State University FHSU Scholars Repository Master's Theses Graduate School Summer 2011 Grief Support Groups: Preference For Online Vs. Face To Face Kris S. Fox Fort Hays State University Follow this and additional works at: https://scholars.fhsu.edu/theses Part of the Psychology Commons Recommended Citation Fox, Kris S., "Grief Support Groups: Preference For Online Vs. Face To Face" (2011). Master's Theses. 144. https://scholars.fhsu.edu/theses/144 This Thesis is brought to you for free and open access by the Graduate School at FHSU Scholars Repository. It has been accepted for inclusion in Master's Theses by an authorized administrator of FHSU Scholars Repository. GRIEF SUPPORT GROUPS: PREFERENCE FOR ONLINE VS. FACE-TO-FACE being A Thesis Presented to the Graduate Faculty of the Fort Hays State University in Partial Fulfillment of the Requirements for the Degree of Master of Science by Kris S. Fox B.S., Fort Hays State University Date_____________________ Approved________________________________ Major Professor Approved________________________________ Chair, Graduate Council ABSTRACT Grief is a reaction to loss and will be experienced to some degree by everyone in his or her life. For most, this is a brief process lasting a few weeks or months, after which they regain their focus and return to their normal lives. For a percentage of the population, however, it is more difficult to return to normal life functions. The grieving process can further diminish low social support and social support networks. However, generally providing the opportunity to talk about their feelings is sufficient to help most work through their grief without therapy (Burke, Eakes, and Hainsworth, 1999; Neimeyer, 2008).