Checklist of Helminths from Lizards and Amphisbaenians (Reptilia, Squamata) of South America Ticle R A
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
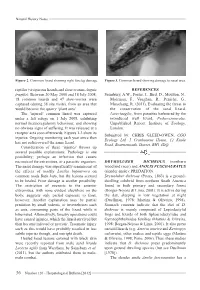
Reptiles (Viviparous Lizards and Slow-Worms Anguis REFERENCES Fragilis)
Natural History Notes Figure 2. Common lizard showing right foreleg damage. Figure 3. Common lizard showing damage to nasal area. reptiles (viviparous lizards and slow-worms Anguis REFERENCES fragilis). Between 20 May 2008 and 18 July 2008, Sainsbury, A.W., Foster, J., Bird, D., Moulton, N., 18 common lizards and 47 slow-worms were Molenaar, F., Vaughan, R., Peniche, G., captured (during 38 site visits), from an area that Marschang, R. (2011). Evaluating the threat to would become the quarry ‘plant area’. the conservation of the sand lizard, The ‘injured’ common lizard was captured Lacertaagilis, from parasites harboured by the under a felt refuge on 1 July 2008, exhibiting introduced wall lizard, Podarcismuralis. normal thermoregulatory behaviour, and showing Unpublished Report. Institute of Zoology, no obvious signs of suffering. It was released at a London. receptor area soon afterwards. Figures 1-3 show its Submitted by: CHRIS GLEED-OWEN, CGO injuries. Ongoing monitoring each year since then Ecology Ltd, 5 Cranbourne House, 12 Knole has not rediscovered the same lizard. Road, Bournemouth, Dorset, BH1 4DQ. Consideration of these ‘injuries’ throws up several possible explanations. Pathology is one possibility; perhaps an infection that causes necrosis of the extremities, or a parasitic organism. DRYMOLUBER DICHROUS (northern The nasal damage was superficially reminiscent of woodland racer) and ANOLIS FUSCOAURATUS the effects of toadfly Lucilia bufonivora on (slender anole): PREDATION. common toads Bufo bufo, but the lesions seemed Drymoluber dichrous (Peters, 1863) is a ground- to be healed. Frost damage is another possibility. dwelling colubrid from northern South America The restriction of necrosis to the anterior found in both primary and secondary forest extremities, with none evident elsewhere on the (Borges-Nojosa & Lima, 2001). -

RESEARCH PUBLICATIONS by ZOO ATLANTA STAFF 1978–Present
RESEARCH PUBLICATIONS BY ZOO ATLANTA STAFF 1978–Present This listing may be incomplete, and some citation information may be incomplete or inaccurate. Please advise us if you are aware of any additional publications. Copies of publications may be available directly from the authors, or from websites such as ResearchGate. Zoo Atlanta does not distribute copies of articles on behalf of these authors. Updated: 22 Jan 2020 1978 1. Maple, T.L., and E.L. Zucker. 1978. Ethological studies of play behavior in captive great apes. In E.O. Smith (Ed.), Social Play in Primates. New York: Academic Press, 113–142. 1979 2. Maple, T.L. 1979. Great apes in captivity: the good, the bad, and the ugly. In J. Erwin, T.L. Maple, G. Mitchell (Eds.), Captivity and Behavior: Primates in Breeding Colonies, Laboratories and Zoos. New York: Van Nostrand Reinhold, 239–272. 3. Strier, K.B., J. Altman, D. Brockman, A. Bronikowski, M. Cords, L. Fedigan, H. Lapp, J. Erwin, T.L. Maple, and G. Mitchell (Eds.). 1979. Captivity and Behavior: Primates in Breeding Colonies, Laboratories and Zoos. New York: Van Nostrand Reinhold, 286. 1981 4. Hoff, M.P., R.D. Nadler, and T.L. Maple. 1981. Development of infant independence in a captive group of lowland gorillas. Developmental Psychobiology 14:251–265. 5. Hoff, M.P., R.D. Nadler, and T.L. Maple. 1981. The development of infant play in a captive group of lowland gorillas (Gorilla gorilla gorilla). American Journal of Primatology 1:65–72. 1982 6. Hoff, M.P., R.D. Nadler, and T.L. Maple. -

Herpetological Information Service No
Type Descriptions and Type Publications OF HoBART M. Smith, 1933 through June 1999 Ernest A. Liner Houma, Louisiana smithsonian herpetological information service no. 127 2000 SMITHSONIAN HERPETOLOGICAL INFORMATION SERVICE The SHIS series publishes and distributes translations, bibliographies, indices, and similar items judged useful to individuals interested in the biology of amphibians and reptiles, but unlikely to be published in the normal technical journals. Single copies are distributed free to interested individuals. Libraries, herpetological associations, and research laboratories are invited to exchange their publications with the Division of Amphibians and Reptiles. We wish to encourage individuals to share their bibliographies, translations, etc. with other herpetologists through the SHIS series. If you have such items please contact George Zug for instructions on preparation and submission. Contributors receive 50 free copies. Please address all requests for copies and inquiries to George Zug, Division of Amphibians and Reptiles, National Museum of Natural History, Smithsonian Institution, Washington DC 20560 USA. Please include a self-addressed mailing label with requests. Introduction Hobart M. Smith is one of herpetology's most prolific autiiors. As of 30 June 1999, he authored or co-authored 1367 publications covering a range of scholarly and popular papers dealing with such diverse subjects as taxonomy, life history, geographical distribution, checklists, nomenclatural problems, bibliographies, herpetological coins, anatomy, comparative anatomy textbooks, pet books, book reviews, abstracts, encyclopedia entries, prefaces and forwords as well as updating volumes being repnnted. The checklists of the herpetofauna of Mexico authored with Dr. Edward H. Taylor are legendary as is the Synopsis of the Herpetofalhva of Mexico coauthored with his late wife, Rozella B. -

Karyological Study of Amphisbaena Ridleyi (Squamata, Amphisbaenidae), an Endemic Species of the Archipelago of Fernando De Noronha, Pernambuco, Brazil
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by PubMed Central Genetics and Molecular Biology, 33, 1, 57-61 (2010) Copyright © 2009, Sociedade Brasileira de Genética. Printed in Brazil www.sbg.org.br Short Communication Karyological study of Amphisbaena ridleyi (Squamata, Amphisbaenidae), an endemic species of the Archipelago of Fernando de Noronha, Pernambuco, Brazil Marcia Maria Laguna1, Renata Cecília Amaro2, Tamí Mott3, Yatiyo Yonenaga-Yassuda1 and Miguel Trefaut Rodrigues2 1Departamento de Genética e Biologia Evolutiva, Instituto de Biociências, Universidade de São Paulo, São Paulo, SP, Brazil. 2Departamento de Zoologia, Instituto de Biociências, Universidade de São Paulo, São Paulo, SP, Brazil. 3Instituto de Biociências, Programa de Pós Graduação em Ecologia e Conservação da Biodiversidade, Universidade Federal do Mato Grosso, Cuiabá, MT, Brazil. Abstract The karyotype of Amphisbaena ridleyi, an endemic species of the archipelago of Fernando de Noronha, in State of Pernambuco, Brazil, is described after conventional staining, Ag-NOR impregnation and fluorescence in situ hybrid- ization (FISH) with a telomeric probe. The diploid number is 46, with nine pairs of macrochromosomes (three metacentrics, four subtelocentrics and two acrocentrics) and 14 pairs of microchromosomes. The Ag-NOR is located in the telomeric region of the long arm of metacentric chromosome 2 and FISH revealed signals only in the telomeric region of all chromosomes. Further cytogenetic data on other amphisbaenians as well as a robust phylogenetic hy- pothesis of this clade is needed in order to understand the evolutionary changes on amphisbaenian karyotypes. Key words: Amphisbaena ridleyi, karyotype, Fernando de Noronha, Ag-NOR, FISH with telomeric probes. -

Iii Pontificia Universidad Católica Del
III PONTIFICIA UNIVERSIDAD CATÓLICA DEL ECUADOR FACULTAD DE CIENCIAS EXACTAS Y NATURALES ESCUELA DE CIENCIAS BIOLÓGICAS Un método integrativo para evaluar el estado de conservación de las especies y su aplicación a los reptiles del Ecuador Tesis previa a la obtención del título de Magister en Biología de la Conservación CAROLINA DEL PILAR REYES PUIG Quito, 2015 IV CERTIFICACIÓN Certifico que la disertación de la Maestría en Biología de la Conservación de la candidata Carolina del Pilar Reyes Puig ha sido concluida de conformidad con las normas establecidas; por tanto, puede ser presentada para la calificación correspondiente. Dr. Omar Torres Carvajal Director de la Disertación Quito, Octubre del 2015 V AGRADECIMIENTOS A Omar Torres-Carvajal, curador de la División de Reptiles del Museo de Zoología de la Pontificia Universidad Católica del Ecuador (QCAZ), por su continua ayuda y contribución en todas las etapas de este estudio. A Andrés Merino-Viteri (QCAZ) por su valiosa ayuda en la generación de mapas de distribución potencial de reptiles del Ecuador. A Santiago Espinosa y Santiago Ron (QCAZ) por sus acertados comentarios y correcciones. A Ana Almendáriz por haber facilitado las localidades geográficas de presencia de ciertos reptiles del Ecuador de la base de datos de la Escuela Politécnica Nacional (EPN). A Mario Yánez-Muñoz de la División de Herpetología del Museo Ecuatoriano de Ciencias Naturales del Instituto Nacional de Biodiversidad (DHMECN-INB), por su ayuda y comentarios a la evaluación de ciertos reptiles del Ecuador. A Marcio Martins, Uri Roll, Fred Kraus, Shai Meiri, Peter Uetz y Omar Torres- Carvajal del Global Assessment of Reptile Distributions (GARD) por su colaboración y comentarios en las encuestas realizadas a expertos. -

Borrador De Tesis
UNIVERSIDAD NACIONAL DE TRUJILLO ESCUELA DE POSTGRADO PROGRAMA: DOCTORADO EN CIENCIAS BIOLÓGICAS Estado actual de las poblaciones de Dicrodon guttulatum y Dicrodon holmbergi, en el Santuario Histórico Bosque Pómac y Pampa Tizal, 2007 TESIS PARA OPTAR EL GRADO DE DOCTOR EN CIENCIAS BIOLÓGICAS AUTOR: Ms. C. LUIS ENRIQUE POLLACK VELÁSQUEZ ASESOR: Dr. RADIGUD FERNÁNDEZ ROMERO TRUJILLO – PERÚ 2009 Nº de Registro: …….. i AUTORIDADES Dr. Carlos Sabana Gamarra Rector Dr. Juan César Muro Morey Vicerrector Académico Dr. Orlando Velásquez Benites Vicerrector Administrativo Dr. Sebastián Bustamante Edquén Director de la Escuela de Postgrado Dr. Steban Ilich Zerpa Profesor Secretario de la Escuela de Postgrado Dr. Federico González Veintemilla Director de Sección de Postgrado en Ciencias Biológicas i PRESENTACIÓN Señores miembros del Jurado En cumplimiento con las disposiciones establecidas en el Reglamento de Grados y Títulos de la Universidad Nacional de Trujillo, pongo a vuestra consideración el trabajo de tesis titulado: Estado actual de las poblaciones de Dicrodon guttulatum y Dicrodon holmbergi, en el Santuario Histórico Bosque Pómac y Pampa Tizal, 2007, con el propósito de obtener el Grado de Doctor en Ciencias Biológicas. Espero que vuestro criterio idóneo sepa comprender algunas omisiones involuntarias, en tal sentido me someto a su dictamen. Trujillo, agosto de 2009. _________________________________ Ms. C. Luis Enrique Pollack Velásquez ii DEL ASESOR Dr. RADIGUD FERNÁNDEZ ROMERO, suscribe que la presente tesis ha sido ejecutada de acuerdo a lo establecido en el plan de trabajo y con las debidas orientaciones brindadas al tesista. Respecto al informe, éste ha sido revisado y acoge las sugerencias pertinentes. __________________________________ Dr. RADIGUD FERNÁNDEZ ROMERO Asesor iii DEL JURADO DICTAMINADOR Los profesores que suscriben, miembros del Jurado Dictaminador, declaran que la presente tesis ha sido ejecutada en concordancia con las normas vigentes de la Escuela de Postgrado de la Universidad Nacional de Trujillo. -

HERPETOLOGICAL BULLETIN Number 106 – Winter 2008
The HERPETOLOGICAL BULLETIN Number 106 – Winter 2008 PUBLISHED BY THE BRITISH HERPETOLOGICAL SOCIETY THE HERPETOLOGICAL BULLETIN Contents RESEA R CH AR TICLES Use of transponders in the post-release monitoring of translocated spiny-tailed lizards (Uromastyx aegyptia microlepis) in Abu Dhabi Emirate, United Arab Emirates Pritpal S. Soorae, Judith Howlett and Jamie Samour .......................... 1 Gastrointestinal helminths of three species of Dicrodon (Squamata: Teiidae) from Peru Stephen R. Goldberg and Charles R. Bursey ..................................... 4 Notes on the Natural History of the eublepharid Gecko Hemitheconyx caudicinctus in northwestern Ghana Stephen Spawls ........................................................ 7 Significant range extension for the Central American Colubrid snake Ninia pavimentata (Bocourt 1883) Josiah H. Townsend, J. Micheal Butler, Larry David Wilson, Lorraine P. Ketzler, John Slapcinsky and Nathaniel M. Stewart ..................................... 15 Predation on Italian Newt larva, Lissotriton italicus (Amphibia, Caudata, Salamandridae), by Agabus bipustulatus (Insecta, Coleoptera, Dytiscidae) Luigi Corsetti and Gianluca Nardi........................................ 18 Behaviour, Time Management, and Foraging Modes of a West Indian Racer, Alsophis sibonius Lauren A. White, Peter J. Muelleman, Robert W. Henderson and Robert Powell . 20 Communal egg-laying and nest-sites of the Goo-Eater, Sibynomorphus mikanii (Colubridae, Dipsadinae) in southeastern Brazil Henrique B. P. Braz, Francisco L. Franco -

Diversificação Morfológica E Molecular Em Lagartos Dactyloidae Sul-Americanos
MUSEU PARAENSE EMÍLIO GOELDI UNIVERSIDADE FEDERAL DO PARÁ PROGRAMA DE PÓS-GRADUAÇÃO EM ZOOLOGIA CURSO DE DOUTORADO EM ZOOLOGIA DIVERSIFICAÇÃO MORFOLÓGICA E MOLECULAR EM LAGARTOS DACTYLOIDAE SUL-AMERICANOS ANNELISE BATISTA D’ANGIOLELLA Belém - PA 2015 ANNELISE BATISTA D’ANGIOLELLA DIVERSIFICAÇÃO MORFOLOGICA E MOLECULAR EM LAGARTOS DACTYLOIDAE SUL-AMERICANOS Tese apresenta ao Programa de Pós-Graduação em Zoologia do convênio Universidade Federal do Pará e Museu Paraense Emílio Goeldi, para obtenção do título de doutora em zoologia. Orientadora: Dra. Tereza Cristina Ávila Pires Co-Orientadora: Dra. Ana Carolina Carnaval Belém - PA 2015 “É capaz quem pensa que é capaz.” ii Agradecimento Ao CNPq pela concessão da minha bolsa de pesquisa. A Capes pela Bolsa de Doutorado Sanduiche no exterior. À Teresa Avila-Pires, minha orientadora, por estar sempre disponível para ajudar, escutar e puxar a orelha! A minha co-orientadora Carol Carnaval, por ter me recebido de braços abertos em seu lab e por toda confiança e apoio. A Ana Prudente pelo passe livre à Coleção e sugestões dadas ao trabalho de hemipenis. Ao Tibério Burlamaqui por toda a ajuda com as análises moleculares e momentos de descontração! A todo o pessoal do laboratório de Herpetologia do MPEG pela companhia e troca de ideias, sempre ajudando quando possível. Ao lab de molecular que foi a minha casa nesses últimos quatro anos e a todos que por ele passaram e contribuíram de alguma forma com meu conhecimento, em especial a Áurea, Geraldo, e Joice. Aos meus filhos de quatro patas Pukey e Bingo por me amarem incondicionalmente. A dança, por ser meu refúgio e por não ter me deixado pirar! Ao meu amor, Bruno, por me inspirar diariamente a ser uma pessoa melhor! Por me impulsionar a ir além e por simplesmente existir em minha vida.. -

De Los Reptiles Del Yasuní
guía dinámica de los reptiles del yasuní omar torres coordinador editorial Lista de especies Número de especies: 113 Amphisbaenia Amphisbaenidae Amphisbaena bassleri, Culebras ciegas Squamata: Serpentes Boidae Boa constrictor, Boas matacaballo Corallus hortulanus, Boas de los jardines Epicrates cenchria, Boas arcoiris Eunectes murinus, Anacondas Colubridae: Dipsadinae Atractus major, Culebras tierreras cafés Atractus collaris, Culebras tierreras de collares Atractus elaps, Falsas corales tierreras Atractus occipitoalbus, Culebras tierreras grises Atractus snethlageae, Culebras tierreras Clelia clelia, Chontas Dipsas catesbyi, Culebras caracoleras de Catesby Dipsas indica, Culebras caracoleras neotropicales Drepanoides anomalus, Culebras hoz Erythrolamprus reginae, Culebras terrestres reales Erythrolamprus typhlus, Culebras terrestres ciegas Erythrolamprus guentheri, Falsas corales de nuca rosa Helicops angulatus, Culebras de agua anguladas Helicops pastazae, Culebras de agua de Pastaza Helicops leopardinus, Culebras de agua leopardo Helicops petersi, Culebras de agua de Peters Hydrops triangularis, Culebras de agua triángulo Hydrops martii, Culebras de agua amazónicas Imantodes lentiferus, Cordoncillos del Amazonas Imantodes cenchoa, Cordoncillos comunes Leptodeira annulata, Serpientes ojos de gato anilladas Oxyrhopus petolarius, Falsas corales amazónicas Oxyrhopus melanogenys, Falsas corales oscuras Oxyrhopus vanidicus, Falsas corales Philodryas argentea, Serpientes liana verdes de banda plateada Philodryas viridissima, Serpientes corredoras -

Do Worm Lizards Occur in Nebraska? Louis A
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Papers in Herpetology Papers in the Biological Sciences 1993 Do Worm Lizards Occur in Nebraska? Louis A. Somma Florida State Collection of Arthropods, [email protected] Follow this and additional works at: http://digitalcommons.unl.edu/biosciherpetology Part of the Biodiversity Commons, and the Population Biology Commons Somma, Louis A., "Do Worm Lizards Occur in Nebraska?" (1993). Papers in Herpetology. 11. http://digitalcommons.unl.edu/biosciherpetology/11 This Article is brought to you for free and open access by the Papers in the Biological Sciences at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in Papers in Herpetology by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. @ o /' number , ,... :S:' .' ,. '. 1'1'13 Do Mono Li ••rel,. Occur ill 1!I! ..br .... l< .. ? by Louis A. Somma Department of- Zoology University of Florida Gainesville, FL 32611 Amphisbaenids, or worm lizards, are a small enigmatic suborder of reptiles (containing 4 families; ca. 140 species) within the order Squamata, which include~ the more speciose lizards and snakes (Gans 1986). The name amphisbaenia is derived from the mythical Amphisbaena (Topsell 1608; Aldrovandi 1640), a two-headed beast (one head at each end), whose fantastical description may have been based, in part, upon actual observations of living worm lizards (Druce 1910). While most are limbless and worm-like in appearance, members of the family Bipedidae (containing the single genus Sipes) have two forelimbs located close to the head. This trait, and the lack of well-developed eyes, makes them look like two-legged worms. -

Supplemental Material Conservation Status of the Herpetofauna
Official journal website: Amphibian & Reptile Conservation amphibian-reptile-conservation.org 8(2) [Special Section]: 1–18; S1–S24 (e87). Supplemental Material Conservation status of the herpetofauna, protected areas, and current problems in Valle del Cauca, Colombia 1Alejandro Valencia-Zuleta, Andrés Felipe Jaramillo-Martínez, Andrea Echeverry-Bocanegra, Ron- ald Viáfara-Vega, Oscar Hernández-Córdoba, Victoria E. Cardona-Botero, Jaime Gutiérrez-Zúñiga, and Fernando Castro-Herrera Universidad del Valle, Grupo Laboratorio de Herpetología, Departamento de Biología, Cali, COLOMBIA Citation: Valencia-Zuleta A, Jaramillo-Martínez AF, Echeverry-Bocanegra A, Viáfara-Vega R, Hernández-Córdoba O, Cardona-Botero VE, Gutiérrez- Zúñiga J, Castro-Herrera F. 2014. Conservation status of the herpetofauna, protected areas, and current problems in Valle del Cauca, Colombia. Amphibian & Reptile Conservation 8(2) [Special Section]: 1–18; S1–S24 (e87). Copyright: © 2014 Valencia-Zuleta et al. This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCom- mercial-NoDerivatives 4.0 International License, which permits unrestricted use for non-commercial and education purposes only, in any medium, provided the original author and the official and authorized publication sources are recognized and properly credited. The official and authorized publication credit sources, which will be duly enforced, are as follows: official journal title Amphibian & Reptile Conservation; official journal website <amphibian-reptile-conservation.org>. Received: 12 March 2014; Accepted: 24 November 2014; Published: 19 December 2014 Table 1. Taxonomic list of amphibians and reptile of the department of Valle del Cauca (Cardona-B. et al. 2014). Actualization of threat categories based on: IUCN (red list), Red Book of Amphibians (Rueda et al. -

Ruínas E Urubus: História Da Ornitologia No Paraná. Período De Natterer, 1 (1820 a 1834) ; Por Fernando C
Hori Cadernos Técnicos 5 RUÍNAS E URUBUS: HISTÓRIA DA ORNITOLOGIA NO PARANÁ PERÍODO DE NATTERER, 1 (1820 a 1834) 1a Edição Fernando C. Straube Hori Consultoria Ambiental Curitiba, Paraná, Brasil Setembro de 2012 © URBEN-FILHO & STRAUBE CONSULTORES S/S LTDA. Ficha catalográfica preparada por DIONE SERIPIERRI (Museu de Zoologia, USP) Straube, Fernando C. Ruínas e urubus: história da ornitologia no Paraná. Período de Natterer, 1 (1820 a 1834) ; por Fernando C. Straube, apresentação de Renato S. Bérnils. – Curitiba, Pr: Hori Consultoria Ambiental, 2012. 241p. (Hori Cadernos Técnicos n. 5) ISBN 978-85-62546-05-1 1. Aves - Paraná. 2. Paraná - Ornitologia. 3. Ornitologia – História. I. Straube, Fernando C. II. Bérnils, Renato S., apresent. II. Título. III. Série. Depósito Legal na Biblioteca Nacional, conforme Decreto n1825, de 20 de dezembro de 1907. Dados internacionais de Catalogação da Publicação (Câmara Brasileira do Livro, São Paulo, Brasil) Capa: Composição com mata de araucária na Lapa (Paraná) (Foto: F.C.Straube), documentos e imagens de autoria de Aimée Adrien Taunay, Michael Sandler, Auguste de Saint-Hilaire e Jean Baptiste Debret, citados no texto. Foto em destaque: urubu-rei (Sarcoramphus papa) de Cassiano “Zapa” Zaparoli Zaniboni (www.zapa.photoshelter.com). 2012 http://www.hori.bio.br HORI CADERNOS TÉCNICOS n° 5 ISBN: 978-85-62546-05-1 CURITIBA, SETEMBRO DE 2012 CITAÇÃO RECOMENDADA Straube, F.C. 2012. Ruínas e urubus: História da Ornitologia no Paraná. Período de Natterer, 1 (1820 a 1834). Curitiba, Hori Consultoria Ambiental. Hori Cadernos Técnicos n° 5, 241+xiii pp. ABERTURA O CENTENÁRIO DA ORNITOLOGIA PARANAENSE “Às minhas viagens ao Paraná, atribuo a importância para que a continuidade do trabalho polonês na América do Sul não seja interrompida.