Quantum Mechanics.Pdf
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
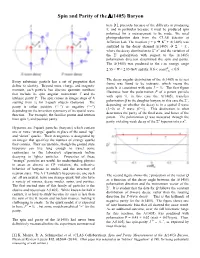
Spin and Parity of the Λ(1405) Baryon
Spin and Parity of the (1405) Baryon here [1], precisely because of the difficulty in producing it, and in particular because it must be produced spin polarized for a measurement to be made. We used photoproduction data from the CLAS detector at Jefferson Lab. The reaction + p K+ + (1405) was analyzed in the decay channel (1405) + + , where the decay distribution to + and the variation of the + polarization with respect to the (1405) polarization direction determined the spin and parity. The (1405) was produced in the c.m. energy range K 2.55 < W < 2.85 GeV and for 0.6 coscm.. 0.9 . The decay angular distribution of the (1405) in its rest Every subatomic particle has a set of properties that frame was found to be isotropic, which means the define its identity. Beyond mass, charge, and magnetic particle is consistent with spin J = ½. The first figure moment, each particle has discrete quantum numbers illustrates how the polarization P of a parent particle that include its spin angular momentum J and its with spin ½, in this case the (1405), transfers intrinsic parity P. The spin comes in integer steps of + starting from ½ for 3-quark objects (baryons). The polarization Q to the daughter baryon, in this case the , depending on whether the decay is in a spatial S wave parity is either positive (“+”) or negative (“”) (L=0) or P wave (L=1). This distinction is what depending on the inversion symmetry of its spatial wave determines the parity of the final state, and hence of the function. For example, the familiar proton and neutron parent. -

The Subatomic Particle Mass Spectrum
The Subatomic Particle Mass Spectrum Robert L. Oldershaw 12 Emily Lane Amherst, MA 01002 USA [email protected] Key Words: Subatomic Particles; Particle Mass Spectrum; General Relativity; Kerr Metric; Discrete Self-Similarity; Discrete Scale Relativity 1 Abstract: Representative members of the subatomic particle mass spectrum in the 100 MeV to 7,000 MeV range are retrodicted to a first approximation using the Kerr solution of General Relativity. The particle masses appear to form a restricted set of quantized values of a Kerr- based angular momentum-mass relation: M = n1/2 M, where values of n are a set of discrete integers and M is a revised Planck mass. A fractal paradigm manifesting global discrete self- similarity is critical to a proper determination of M, which differs from the conventional Planck mass by a factor of roughly 1019. This exceedingly simple and generic mass equation retrodicts the masses of a representative set of 27 well-known particles with an average relative error of 1.6%. A more rigorous mass formula, which includes the total spin angular momentum rule of Quantum Mechanics, the canonical spin values of the particles, and the dimensionless rotational parameter of the Kerr angular momentum-mass relation, is able to retrodict the masses of the 8 dominant baryons in the 900 MeV to 1700 MeV range at the < 99.7% > level. 2 “There remains one especially unsatisfactory feature [of the Standard Model of particle physics]: the observed masses of the particles, m. There is no theory that adequately explains these numbers. We use the numbers in all our theories, but we do not understand them – what they are, or where they come from. -

FUNDAMENTAL PARTICLES Year 14 Physics Erin Hannigan
FUNDAMENTAL PARTICLES Year 14 Physics Erin Hannigan 1 Key Word List ■ Natural Philosophy – the science of matter and energy and their interactions. ■ Hadron – any elementary particle that interacts strongly with other particles. ■ Lepton – an elementary particle that participates in weak interactions. ■ Subatomic Particle – a body having finite mass and internal structure but negligible dimensions. ■ Quark – any of a number f subatomic particles carrying a fractional electric charge, postulated as building blocks of the hadrons. Quarks have not been directly observed but theoretical predictions based on their existence have been confirmed experimentally. ■ Atom – the smallest component of an element having the chemical properties of the element. 2 Fundamental Particles ■ Fundamental particles (also called elementary particles) are the smallest building blocks of the universe. The key characteristic of fundamental particles is that they have no internal structure. ■ There are two type of fundamental particles: – Particles that make up all matter, called fermions – Particles that carry force, called bosons ■ The four fundamental forces include: – Gravity – The weak force – Electromagnetism – The strong force 3 The Four Fundamental Forces ■ The four fundamental forces of nature govern everything that happens in the universe. ■ Gravity – The attraction between two objects that have mass or energy ■ The weak force – Responsible for particle decay – Physicists describe this interaction through the exchange of force-carrying particles called -

Introduction to Subatomic- Particle Spectrometers∗
IIT-CAPP-15/2 Introduction to Subatomic- Particle Spectrometers∗ Daniel M. Kaplan Illinois Institute of Technology Chicago, IL 60616 Charles E. Lane Drexel University Philadelphia, PA 19104 Kenneth S. Nelsony University of Virginia Charlottesville, VA 22901 Abstract An introductory review, suitable for the beginning student of high-energy physics or professionals from other fields who may desire familiarity with subatomic-particle detection techniques. Subatomic-particle fundamentals and the basics of particle in- teractions with matter are summarized, after which we review particle detectors. We conclude with three examples that illustrate the variety of subatomic-particle spectrom- eters and exemplify the combined use of several detection techniques to characterize interaction events more-or-less completely. arXiv:physics/9805026v3 [physics.ins-det] 17 Jul 2015 ∗To appear in the Wiley Encyclopedia of Electrical and Electronics Engineering. yNow at Johns Hopkins University Applied Physics Laboratory, Laurel, MD 20723. 1 Contents 1 Introduction 5 2 Overview of Subatomic Particles 5 2.1 Leptons, Hadrons, Gauge and Higgs Bosons . 5 2.2 Neutrinos . 6 2.3 Quarks . 8 3 Overview of Particle Detection 9 3.1 Position Measurement: Hodoscopes and Telescopes . 9 3.2 Momentum and Energy Measurement . 9 3.2.1 Magnetic Spectrometry . 9 3.2.2 Calorimeters . 10 3.3 Particle Identification . 10 3.3.1 Calorimetric Electron (and Photon) Identification . 10 3.3.2 Muon Identification . 11 3.3.3 Time of Flight and Ionization . 11 3.3.4 Cherenkov Detectors . 11 3.3.5 Transition-Radiation Detectors . 12 3.4 Neutrino Detection . 12 3.4.1 Reactor Neutrinos . 12 3.4.2 Detection of High Energy Neutrinos . -

The Ghost Particle 1 Ask Students If They Can Think of Some Things They Cannot Directly See but They Know Exist
Original broadcast: February 21, 2006 BEFORE WATCHING The Ghost Particle 1 Ask students if they can think of some things they cannot directly see but they know exist. Have them provide examples and reasoning for PROGRAM OVERVIEW how they know these things exist. NOVA explores the 70–year struggle so (Some examples and evidence of their existence include: [bacteria and virus- far to understand the most elusive of all es—illnesses], [energy—heat from the elementary particles, the neutrino. sun], [magnetism—effect on a com- pass], and [gravity—objects falling The program: towards Earth].) How do scientists • relates how the neutrino first came to be theorized by physicist observe and measure things that cannot be seen with the naked eye? Wolfgang Pauli in 1930. (They use instruments such as • notes the challenge of studying a particle with no electric charge. microscopes and telescopes, and • describes the first experiment that confirmed the existence of the they look at how unseen things neutrino in 1956. affect other objects.) • recounts how scientists came to believe that neutrinos—which are 2 Review the structure of an atom, produced during radioactive decay—would also be involved in including protons, neutrons, and nuclear fusion, a process suspected as the fuel source for the sun. electrons. Ask students what they know about subatomic particles, • tells how theoretician John Bahcall and chemist Ray Davis began i.e., any of the various units of mat- studying neutrinos to better understand how stars shine—Bahcall ter below the size of an atom. To created the first mathematical model predicting the sun’s solar help students better understand the neutrino production and Davis designed an experiment to measure size of some subatomic particles, solar neutrinos. -

Make an Atom Vocabulary Grade Levels
MAKE AN ATOM Fundamental to physical science is a basic understanding of the atom. Atoms are comprised of protons, neutrons, and electrons. Protons and neutrons are at the center of the atom while electrons live in lobe-shaped clouds outside the nucleus. The number of electrons usually matches the number of protons, yielding a net neutral charge for the atom. Sometimes an atom has less neutrons or more neutrons than protons. This is called an isotope. If an atom has different numbers of electrons than protons, then it is an ion. If an atom has different numbers of protons, it is a different element all together. Scientists at Idaho National Laboratory study, create, and use radioactive isotopes like Uranium 234. The 234 means this isotope has an atomic mass of 234 Atomic Mass Units (AMU). GRADE LEVELS: 3-8 VOCABULARY Atom – The basic unit of a chemical element. Proton – A stable subatomic particle occurring in all atomic nuclei, with a positive electric charge equal in magnitude to that of an electron, but of opposite sign. Neutron – A subatomic particle of about the same mass as a proton but without an electric charge, present in all atomic nuclei except those of ordinary hydrogen. Electron – A stable subatomic particle with a charge of negative electricity, found in all atoms and acting as the primary carrier of electricity in solids. Orbital – Each of the actual or potential patterns of electron density that may be formed on an atom or molecule by one or more electrons. Ion – An atom or molecule with a net electric charge due to the loss or gain of one or more electrons. -

Classification of Elementary Particles
CLASSIFICATION OF ELEMENTARY PARTICLES #Classification On the basis of “SPIN”: - •Elementary Particles are categorised in two classes i.e. Bosons and Fermions: - BOSONS Introduction: In particle physics boson is the type of particle that obeys the rule of Bose- Einstein statistics. These bosons have quantum spin having integral value 0, +1, -1, +2, -2, etc. •History: The name Boson comes from the surname of Indian physicist Satyendra Nath Bose and physicist Albert Einstein. They develop a method of analysis called Bose-Einstein Statistics. In an effort to understand the Plank's law, Bose proposed a method to analyse the behaviour of photon in 1924.He sent paper to Einstein. One of the most dramatic effect of Bose- Einstein Statistics is the prediction that boson can overlap and coexist with other bosons. Because of this it is possible to become a photon to laser. The name Boson First coined by scientists Paul Dirac in the honour of famous scientists Satyendra Nath Bose and Albert Einstein. Regarding: •Boson are fundamental particle such as gluons ,W ,Z boson, recently discovered Higgs boson aka God particle and Graviton. •Boson may be elementary like Photon or Composite i.e.made with two or more elementary particle. •All observed particles are either fermion or Boson. Types of Bosons- •Bosons are categorised into two categories; Gauge Boson: Gauge Boson are the particle that Carries the interaction of fundamental forces between particles. All known Gauge Boson have spin 1 and therefore all Gauge Boson are vector. •gauge boson is characterized into 5 categories. •Gluons(g): That carries strong nuclear forces between matter particle. -

Thesis Submitted to the Department of Physics in Partial Fulfillment of the Requirements for Graduation with Honors in the Major
THE FLORIDA STATE UNIVERSITY COLLEGE OF ARTS & SCIENCES W BOSON PRODUCTION CHARGE ASYMMETRY IN THE ELECTRON CHANNEL By ASHLEY S HUFF A Thesis submitted to the Department of Physics In partial fulfillment of the requirements for graduation with Honors in the Major Degree Awarded: Spring, 2013 2 The members of the Defense Committee approve the thesis of Ashley Huff defended on April 23, 2013 ____________________________________ Dr. Susan Blessing Thesis Director ____________________________________ Dr. Paul Cottle Committee Member ____________________________________ Dr. Ewa Bienkiewicz Outside Committee Member 3 Abstract We present a measurement of the boson production charge asymmetry in collisions in the electron channel through decays using data collected with the DØ detector. The collision of a quark with a quark can produce a boson while the collision of a quark and a quark can produce a boson. These particles decay rapidly but we are able to measure their asymmetry by studying the resulting final state electrons and neutrinos. These results will be used to further constrain fits to parton distribution functions (PDFs) and improve the accuracy of future predictions for new physics. 4 Table of Contents I. Introduction A. The Standard Model B. W boson production C. The Detector and Data Collection II. Analysis A. Selection Cuts B. Transverse Mass Reconstruction C. Raw asymmetry D. Weighted average asymmetry E. Z boson and Charge Correction F. Efficiencies G. Fake Rate H. Solenoid Polarity I. New Data: RunIIB J. Monte Carlo III. Conclusion IV. References 5 I. Introduction A. The Standard Model The Standard Model of particle physics is the theory that explains the fundamental particles and the interactions between them. -

Evidence of Exotic Meson Particle Discovered At
Vol. 51 - No. 37 September 19, 1997 BROOKHAVEN NATIONAL LABORATORY Evidence of Exotic Meson Particle Discovered at AGS Researchers at BNL’s Alternating mass of 1.4 GeV after years of select- Gradient Synchrotron (AGS) accelera- ing and analyzing data from products tor have found evidence of a new and of billions of particle collisions made rare subatomic particle called an “ex- during the experiment’s 1994 run. otic” meson. The result was presented to par- This finding at AGS Experiment ticipants of the Seventh International 852, described in the September 1 Conference on Hadron Spectroscopy, issue of Physical Review Letters, helps held at BNL August 25-30 (see story validate the standard model, the cen- on page 2). At the conference, confir- tral theory of modern physics. mation of the E852 result was re- Also, the finding could improve un- ported by what is called the Crystal derstanding of how matter binds to- Barrel collaboration at CERN, the gether at the relatively low energy European particle physics laboratory. level of 18 billion electron volts (GeV). “In some sense, we’ve discovered a The E852 team of 51 scientists, new form of matter,” said Chung. “This graduate students and undergradu- particle had been hypothesized [in ates includes researchers from: BNL, the late 1970s], but it’s never, never Northwestern University, Rensselaer been pinned down. Polytechnic Institute, the University “To find evidence of a particle that of Massachusetts, Dartmouth College, has never been detected before, one and Russia’s Institute for High En- that’s so important to our understand- ergy Physics and the Moscow State ing of elementary particles, is hugely University. -

New Subatomic Particle Discovered
New Subatomic Particle Discovered A new subatomic particle has just been discovered by the CLEO elementary particle physics experiment, a collaboration of 150 physicists from sixteen North American Universities The collaboration, of which the Purdue Elementary Particle Physics group is a leading member, is based at Cornell University s particle accelerator the Electron Storage Ring CESR. At CESR counter rotating beams of electrons and positrons (the anti matter counterpart of the electron) collide and annihilate producing quarks, which are one of the fundamental building blocks of all matter. The collaboration has announced the discovery of a new subatomic particle dubbed the D_{sJ}(2463) pronounced: "D sub sJ 2463". This particle, and another, the D^*_{sJ}(2317), discovered in April by the BaBar experiment at the Stanford Linear Accelerator Center, and confirmed by CLEO in the same announcement, were completely unexpected. A proton is made of two up quarks and a down quark. The new particles, which have masses about 2.5 times the mass of a proton, are believed to consist of a quark atom in which a heavy charm quark and a lighter anti strange quark are rotating about one another held together by the strong nuclear force. The co-Spokespersons of the CLEO Collaboration are Purdue Professor Ian Shipsey and Cornell Professor David Cassel. Other collaboration members include Purdue Professors David Miller (a former CLEO co-spokesperson) and Edward Shibata, post doctoral research associates Guangshun Huang and Victor Pavlunin, and graduate student Batbold Sanghi. Shipsey said: The discoveries can be understood in terms of Heavy Quark Effective Theory (HQET) which was developed in the early nineties to describe the behavior of quark anti-quark systems where one quark is much heavier than the other, in combination with a postulated fundamental symmetry in nature between subatomic particles containing heavy quarks called chiral or left-right symmetry. -

S 1-5 1. Define Each of the Following: A. Atom B. Electron C. Nucleus D
Page 74 #’s 1-5 1. Define each of the following: a. atom b. electron c. nucleus d. proton e. neutron a. the smallest piece of an element that is still that element b. a negative part of the atom which give the atom its volume c. the positively charged, extremely dense center part of an atom that contains nearly all the atom's mass but takes up a extremely small part of its volume d. the subatomic particle that has a positive charge equal (in size) to the negative charge of an electron; found in the nucleus e. the electrically neutral subatomic particle found in atomic nuclei 2. Describe one conclusion made by each of the following scientists that led to the development of the current atomic theory: a. Thomson b. Millikan c. Rutherford a. concluded that all atoms have charged parts some positive, some negative b. confirmed the negative charge of the electron and suggested the mass of an electron is ~ 1/2000 of the mass of a hydrogen atom c. determined that most of the mass of an atom is found in the nucleus and that the nucleus occupies very little space within an atom 3. Compare and contrast the three types of subatomic particles in terms of location in the atom, mass, and relative charge. Electrons surround the nucleus and have a negative charge and small mass relative to protons and neutrons Most of an atom's mass Is In its nucleus, which is near the center and is composed of protons and neutrons Protons have a positive charge; neutrons have no charge. -

Particle Prediction
Particle prediction Computer simulation of an event in which the decay of a Higgs boson produces four muons. Photo credit: CERN/Photo Researchers, Inc. For years, physicists have been trying to see and measure subatomic particles and describe their actual energy, momentum, mass, charge, motion, life time, and spin. Recently, evidence of the existence of a long sought after subatomic particle, the Higgs boson, has been confirmed. A way of seeing these tiny particles, or at least traces of where they have just been, is by moving tiny protons towards each other at high speeds and observing what particles are emitted when they collide. Imagine for a moment two racecars (unmanned) heading towards each other at 150 mph. Their collision would have the force of both of their speeds combined, 300 mph, and the race cars would, no doubt, break into smaller pieces. The same idea is what is driving the present experiments in physics … to try to visualize and measure the tiny particles that are emitted after colliding protons at immense speed. A gigantic machine has been built partially in France and partially in Switzerland to create these collisions. Near Geneva, buried beneath the earth, there is the largest and highest energy particle accelerator in the world. The European Laboratory for Particle Physics (CERN) has created with 111 nations and thousands of scientists, the Large Hadron Collider (LHC) to try to observe and measure subatomic particles. The LHC lies in a tunnel 17 miles in circumference, as deep as 574 feet beneath the France/Swiss border near Geneva, Switzerland.