S 1-5 1. Define Each of the Following: A. Atom B. Electron C. Nucleus D
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
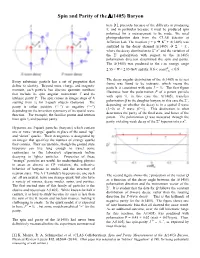
Spin and Parity of the Λ(1405) Baryon
Spin and Parity of the (1405) Baryon here [1], precisely because of the difficulty in producing it, and in particular because it must be produced spin polarized for a measurement to be made. We used photoproduction data from the CLAS detector at Jefferson Lab. The reaction + p K+ + (1405) was analyzed in the decay channel (1405) + + , where the decay distribution to + and the variation of the + polarization with respect to the (1405) polarization direction determined the spin and parity. The (1405) was produced in the c.m. energy range K 2.55 < W < 2.85 GeV and for 0.6 coscm.. 0.9 . The decay angular distribution of the (1405) in its rest Every subatomic particle has a set of properties that frame was found to be isotropic, which means the define its identity. Beyond mass, charge, and magnetic particle is consistent with spin J = ½. The first figure moment, each particle has discrete quantum numbers illustrates how the polarization P of a parent particle that include its spin angular momentum J and its with spin ½, in this case the (1405), transfers intrinsic parity P. The spin comes in integer steps of + starting from ½ for 3-quark objects (baryons). The polarization Q to the daughter baryon, in this case the , depending on whether the decay is in a spatial S wave parity is either positive (“+”) or negative (“”) (L=0) or P wave (L=1). This distinction is what depending on the inversion symmetry of its spatial wave determines the parity of the final state, and hence of the function. For example, the familiar proton and neutron parent. -

The Subatomic Particle Mass Spectrum
The Subatomic Particle Mass Spectrum Robert L. Oldershaw 12 Emily Lane Amherst, MA 01002 USA [email protected] Key Words: Subatomic Particles; Particle Mass Spectrum; General Relativity; Kerr Metric; Discrete Self-Similarity; Discrete Scale Relativity 1 Abstract: Representative members of the subatomic particle mass spectrum in the 100 MeV to 7,000 MeV range are retrodicted to a first approximation using the Kerr solution of General Relativity. The particle masses appear to form a restricted set of quantized values of a Kerr- based angular momentum-mass relation: M = n1/2 M, where values of n are a set of discrete integers and M is a revised Planck mass. A fractal paradigm manifesting global discrete self- similarity is critical to a proper determination of M, which differs from the conventional Planck mass by a factor of roughly 1019. This exceedingly simple and generic mass equation retrodicts the masses of a representative set of 27 well-known particles with an average relative error of 1.6%. A more rigorous mass formula, which includes the total spin angular momentum rule of Quantum Mechanics, the canonical spin values of the particles, and the dimensionless rotational parameter of the Kerr angular momentum-mass relation, is able to retrodict the masses of the 8 dominant baryons in the 900 MeV to 1700 MeV range at the < 99.7% > level. 2 “There remains one especially unsatisfactory feature [of the Standard Model of particle physics]: the observed masses of the particles, m. There is no theory that adequately explains these numbers. We use the numbers in all our theories, but we do not understand them – what they are, or where they come from. -

FUNDAMENTAL PARTICLES Year 14 Physics Erin Hannigan
FUNDAMENTAL PARTICLES Year 14 Physics Erin Hannigan 1 Key Word List ■ Natural Philosophy – the science of matter and energy and their interactions. ■ Hadron – any elementary particle that interacts strongly with other particles. ■ Lepton – an elementary particle that participates in weak interactions. ■ Subatomic Particle – a body having finite mass and internal structure but negligible dimensions. ■ Quark – any of a number f subatomic particles carrying a fractional electric charge, postulated as building blocks of the hadrons. Quarks have not been directly observed but theoretical predictions based on their existence have been confirmed experimentally. ■ Atom – the smallest component of an element having the chemical properties of the element. 2 Fundamental Particles ■ Fundamental particles (also called elementary particles) are the smallest building blocks of the universe. The key characteristic of fundamental particles is that they have no internal structure. ■ There are two type of fundamental particles: – Particles that make up all matter, called fermions – Particles that carry force, called bosons ■ The four fundamental forces include: – Gravity – The weak force – Electromagnetism – The strong force 3 The Four Fundamental Forces ■ The four fundamental forces of nature govern everything that happens in the universe. ■ Gravity – The attraction between two objects that have mass or energy ■ The weak force – Responsible for particle decay – Physicists describe this interaction through the exchange of force-carrying particles called -

Introduction to Subatomic- Particle Spectrometers∗
IIT-CAPP-15/2 Introduction to Subatomic- Particle Spectrometers∗ Daniel M. Kaplan Illinois Institute of Technology Chicago, IL 60616 Charles E. Lane Drexel University Philadelphia, PA 19104 Kenneth S. Nelsony University of Virginia Charlottesville, VA 22901 Abstract An introductory review, suitable for the beginning student of high-energy physics or professionals from other fields who may desire familiarity with subatomic-particle detection techniques. Subatomic-particle fundamentals and the basics of particle in- teractions with matter are summarized, after which we review particle detectors. We conclude with three examples that illustrate the variety of subatomic-particle spectrom- eters and exemplify the combined use of several detection techniques to characterize interaction events more-or-less completely. arXiv:physics/9805026v3 [physics.ins-det] 17 Jul 2015 ∗To appear in the Wiley Encyclopedia of Electrical and Electronics Engineering. yNow at Johns Hopkins University Applied Physics Laboratory, Laurel, MD 20723. 1 Contents 1 Introduction 5 2 Overview of Subatomic Particles 5 2.1 Leptons, Hadrons, Gauge and Higgs Bosons . 5 2.2 Neutrinos . 6 2.3 Quarks . 8 3 Overview of Particle Detection 9 3.1 Position Measurement: Hodoscopes and Telescopes . 9 3.2 Momentum and Energy Measurement . 9 3.2.1 Magnetic Spectrometry . 9 3.2.2 Calorimeters . 10 3.3 Particle Identification . 10 3.3.1 Calorimetric Electron (and Photon) Identification . 10 3.3.2 Muon Identification . 11 3.3.3 Time of Flight and Ionization . 11 3.3.4 Cherenkov Detectors . 11 3.3.5 Transition-Radiation Detectors . 12 3.4 Neutrino Detection . 12 3.4.1 Reactor Neutrinos . 12 3.4.2 Detection of High Energy Neutrinos . -

The Ghost Particle 1 Ask Students If They Can Think of Some Things They Cannot Directly See but They Know Exist
Original broadcast: February 21, 2006 BEFORE WATCHING The Ghost Particle 1 Ask students if they can think of some things they cannot directly see but they know exist. Have them provide examples and reasoning for PROGRAM OVERVIEW how they know these things exist. NOVA explores the 70–year struggle so (Some examples and evidence of their existence include: [bacteria and virus- far to understand the most elusive of all es—illnesses], [energy—heat from the elementary particles, the neutrino. sun], [magnetism—effect on a com- pass], and [gravity—objects falling The program: towards Earth].) How do scientists • relates how the neutrino first came to be theorized by physicist observe and measure things that cannot be seen with the naked eye? Wolfgang Pauli in 1930. (They use instruments such as • notes the challenge of studying a particle with no electric charge. microscopes and telescopes, and • describes the first experiment that confirmed the existence of the they look at how unseen things neutrino in 1956. affect other objects.) • recounts how scientists came to believe that neutrinos—which are 2 Review the structure of an atom, produced during radioactive decay—would also be involved in including protons, neutrons, and nuclear fusion, a process suspected as the fuel source for the sun. electrons. Ask students what they know about subatomic particles, • tells how theoretician John Bahcall and chemist Ray Davis began i.e., any of the various units of mat- studying neutrinos to better understand how stars shine—Bahcall ter below the size of an atom. To created the first mathematical model predicting the sun’s solar help students better understand the neutrino production and Davis designed an experiment to measure size of some subatomic particles, solar neutrinos. -

Chapter 12: Phenomena
Chapter 12: Phenomena Phenomena: Different wavelengths of electromagnetic radiation were directed onto two different metal sample (see picture). Scientists then recorded if any particles were ejected and if so what type of particles as well as the speed of the particle. What patterns do you see in the results that were collected? K Wavelength of Light Where Particles Ejected Speed of Exp. Intensity Directed at Sample Ejected Particle Ejected Particle -7 - 4 푚 1 5.4×10 m Medium Yes e 4.9×10 푠 -8 - 6 푚 2 3.3×10 m High Yes e 3.5×10 푠 3 2.0 m High No N/A N/A 4 3.3×10-8 m Low Yes e- 3.5×106 푚 Electromagnetic 푠 Radiation 5 2.0 m Medium No N/A N/A Ejected Particle -12 - 8 푚 6 6.1×10 m High Yes e 2.7×10 푠 7 5.5×104 m High No N/A N/A Fe Wavelength of Light Where Particles Ejected Speed of Exp. Intensity Directed at Sample Ejected Particle Ejected Particle 1 5.4×10-7 m Medium No N/A N/A -11 - 8 푚 2 3.4×10 m High Yes e 1.1×10 푠 3 3.9×103 m Medium No N/A N/A -8 - 6 푚 4 2.4×10 m High Yes e 4.1×10 푠 5 3.9×103 m Low No N/A N/A -7 - 5 푚 6 2.6×10 m Low Yes e 1.1×10 푠 -11 - 8 푚 7 3.4×10 m Low Yes e 1.1×10 푠 Chapter 12: Quantum Mechanics and Atomic Theory Chapter 12: Quantum Mechanics and Atomic Theory o Electromagnetic Radiation o Quantum Theory o Particle in a Box Big Idea: The structure of atoms o The Hydrogen Atom must be explained o Quantum Numbers using quantum o Orbitals mechanics, a theory in o Many-Electron Atoms which the properties of particles and waves o Periodic Trends merge together. -

Introduction to Chemistry
Introduction to Chemistry Author: Tracy Poulsen Digital Proofer Supported by CK-12 Foundation CK-12 Foundation is a non-profit organization with a mission to reduce the cost of textbook Introduction to Chem... materials for the K-12 market both in the U.S. and worldwide. Using an open-content, web-based Authored by Tracy Poulsen collaborative model termed the “FlexBook,” CK-12 intends to pioneer the generation and 8.5" x 11.0" (21.59 x 27.94 cm) distribution of high-quality educational content that will serve both as core text as well as provide Black & White on White paper an adaptive environment for learning. 250 pages ISBN-13: 9781478298601 Copyright © 2010, CK-12 Foundation, www.ck12.org ISBN-10: 147829860X Except as otherwise noted, all CK-12 Content (including CK-12 Curriculum Material) is made Please carefully review your Digital Proof download for formatting, available to Users in accordance with the Creative Commons Attribution/Non-Commercial/Share grammar, and design issues that may need to be corrected. Alike 3.0 Unported (CC-by-NC-SA) License (http://creativecommons.org/licenses/by-nc- sa/3.0/), as amended and updated by Creative Commons from time to time (the “CC License”), We recommend that you review your book three times, with each time focusing on a different aspect. which is incorporated herein by this reference. Specific details can be found at http://about.ck12.org/terms. Check the format, including headers, footers, page 1 numbers, spacing, table of contents, and index. 2 Review any images or graphics and captions if applicable. -

Atomic Physicsphysics AAA Powerpointpowerpointpowerpoint Presentationpresentationpresentation Bybyby Paulpaulpaul E.E.E
ChapterChapter 38C38C -- AtomicAtomic PhysicsPhysics AAA PowerPointPowerPointPowerPoint PresentationPresentationPresentation bybyby PaulPaulPaul E.E.E. Tippens,Tippens,Tippens, ProfessorProfessorProfessor ofofof PhysicsPhysicsPhysics SouthernSouthernSouthern PolytechnicPolytechnicPolytechnic StateStateState UniversityUniversityUniversity © 2007 Objectives:Objectives: AfterAfter completingcompleting thisthis module,module, youyou shouldshould bebe ableable to:to: •• DiscussDiscuss thethe earlyearly modelsmodels ofof thethe atomatom leadingleading toto thethe BohrBohr theorytheory ofof thethe atom.atom. •• DemonstrateDemonstrate youryour understandingunderstanding ofof emissionemission andand absorptionabsorption spectraspectra andand predictpredict thethe wavelengthswavelengths oror frequenciesfrequencies ofof thethe BalmerBalmer,, LymanLyman,, andand PashenPashen spectralspectral series.series. •• CalculateCalculate thethe energyenergy emittedemitted oror absorbedabsorbed byby thethe hydrogenhydrogen atomatom whenwhen thethe electronelectron movesmoves toto aa higherhigher oror lowerlower energyenergy level.level. PropertiesProperties ofof AtomsAtoms ••• AtomsAtomsAtoms areareare stablestablestable andandand electricallyelectricallyelectrically neutral.neutral.neutral. ••• AtomsAtomsAtoms havehavehave chemicalchemicalchemical propertiespropertiesproperties whichwhichwhich allowallowallow themthemthem tototo combinecombinecombine withwithwith otherotherother atoms.atoms.atoms. ••• AtomsAtomsAtoms emitemitemit andandand absorbabsorbabsorb -

22. Atomic Physics and Quantum Mechanics
22. Atomic Physics and Quantum Mechanics S. Teitel April 14, 2009 Most of what you have been learning this semester and last has been 19th century physics. Indeed, Maxwell's theory of electromagnetism, combined with Newton's laws of mechanics, seemed at that time to express virtually a complete \solution" to all known physical problems. However, already by the 1890's physicists were grappling with certain experimental observations that did not seem to fit these existing theories. To explain these puzzling phenomena, the early part of the 20th century witnessed revolutionary new ideas that changed forever our notions about the physical nature of matter and the theories needed to explain how matter behaves. In this lecture we hope to give the briefest introduction to some of these ideas. 1 Photons You have already heard in lecture 20 about the photoelectric effect, in which light shining on the surface of a metal results in the emission of electrons from that surface. To explain the observed energies of these emitted elec- trons, Einstein proposed in 1905 that the light (which Maxwell beautifully explained as an electromagnetic wave) should be viewed as made up of dis- crete particles. These particles were called photons. The energy of a photon corresponding to a light wave of frequency f is given by, E = hf ; where h = 6:626×10−34 J · s is a new fundamental constant of nature known as Planck's constant. The total energy of a light wave, consisting of some particular number n of photons, should therefore be quantized in integer multiples of the energy of the individual photon, Etotal = nhf. -

Atomic Theory - Review Sheet
Atomic Theory - Review Sheet You should understand the contribution of each scientist. I don't expect you to memorize dates, but I do want to know what each scientist contributed to the theory. Democretus - 400 B.C. - theory that matter is made of atoms Boyle 1622 -1691 - defined element Lavoisier 1743-1797 - pioneer of modern chemistry (careful and controlled experiments) - conservation of mass (understand his experiment and compare to the lab "Does mass change in a chemical reaction?") Proust 1799 - law of constant composition (or definite proportions) - understand this law and relate it to the Hydrogen and Oxygen generation lab Dalton 1808 - Understand the meaning of each part of Dalton's atomic theory. - You don't have to memorize the five parts, just understand what they mean. - You should know which parts we now know are not true (according to modern atomic theory). Black Box Model Crookes 1870 - Crookes tube - Know how this is constructed (Know how cathode rays are generated and what they are.) J.J. Thomsen - 1890 - How did he expand on Crookes' experiment? - discovered the electron - negative particles in all of matter (all atoms) - must also be positive parts Lord Kelvin - near Thomsen's time Plum Pudding Model - plum pudding model Henri Becquerel - 1896 - discovered radioactivity of uranium Marie Curie - 1905 - received Nobel prize (shared with her husband Pierre Curie and Henri Bequerel) for her work in studying radiation. - Name the three kinds of radiation and describe each type. Rutheford - 1910 - Understand the gold foil experiment - How was it set up? - What did it show? - Discovered the proton Hard Nucleus Model - new model of atom (positively charged, very dense nucleus) Niels Bohr - 1913 - Bohr model of the atom - electrons in specific orbits with specific energies James Chadwick - 1932 - discovered the electron Modern version of atom - 1950 Bohr Model - similar nucleus (protons + neutrons) - electrons in orbitals not orbits Bohr Model Understand isotopes, atomic mass, mass #, atomic #, ions (with neutrons) Nature of light - wavelength vs. -

Chapter 11: Modernatomictheory 105
CHAPTER 11 Modern Atomic Theory INTRODUCTION It is difficult to form a mental image of atoms because we can’t see them. Scientists have produced models that account for the behavior of atoms by making observations about the properties of atoms. So we know quite a bit about these tiny particles which make up matter even though we cannot see them. In this chapter you will learn how chemists believe atoms are structured. CHAPTER DISCUSSION This is yet another chapter in which models play a significant role. Recall that we like models to be simple, but we also need models to explain the questions we want to answer. For example, Dalton’s model of the atom has the advantage of being quite simple and is useful when considering molecular- level views of solids, liquids, and gases and in representing chemical equations. However, it cannot explain fundamental questions such as why atoms “stick” together to form molecules. Scientists such as Thomson and Rutherford expanded the model of the atom to include subatomic particles. The model is more complicated than Dalton’s, but it begins to explain chemical reactivity (electrons are involved) and formulas for ionic compounds. But questions still abound. For example, why are there similarities in reactivities of elements (periodic trends)? To begin our understanding of modern atomic theory, let’s first discuss some observations. For example, you have undoubtedly seen a fireworks display, either in person or on television. Where do the colors come from? How do we get so many different colors? It turns out that different salts, when heated, give off different colors. -

Make an Atom Vocabulary Grade Levels
MAKE AN ATOM Fundamental to physical science is a basic understanding of the atom. Atoms are comprised of protons, neutrons, and electrons. Protons and neutrons are at the center of the atom while electrons live in lobe-shaped clouds outside the nucleus. The number of electrons usually matches the number of protons, yielding a net neutral charge for the atom. Sometimes an atom has less neutrons or more neutrons than protons. This is called an isotope. If an atom has different numbers of electrons than protons, then it is an ion. If an atom has different numbers of protons, it is a different element all together. Scientists at Idaho National Laboratory study, create, and use radioactive isotopes like Uranium 234. The 234 means this isotope has an atomic mass of 234 Atomic Mass Units (AMU). GRADE LEVELS: 3-8 VOCABULARY Atom – The basic unit of a chemical element. Proton – A stable subatomic particle occurring in all atomic nuclei, with a positive electric charge equal in magnitude to that of an electron, but of opposite sign. Neutron – A subatomic particle of about the same mass as a proton but without an electric charge, present in all atomic nuclei except those of ordinary hydrogen. Electron – A stable subatomic particle with a charge of negative electricity, found in all atoms and acting as the primary carrier of electricity in solids. Orbital – Each of the actual or potential patterns of electron density that may be formed on an atom or molecule by one or more electrons. Ion – An atom or molecule with a net electric charge due to the loss or gain of one or more electrons.