18 Chapter 2: the Atom an Atom Is the Smallest Particle of an Element
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Confinement and Exotic Meson Spectroscopy at 12Gev JLAB
174 Brazilian Journal of Physics, vol. 33, no. 2, June, 2003 Confinement and Exotic Meson Spectroscopy at 12GeV JLAB Adam Szczepaniak Physics Department, Indiana University, Bloomington, IN 47405, USA Received on 30 October, 2002 Phenomenology of gluonic excitations and possibilities for searches of exotic mesons at JLab are discussed. I Introduction O(104) number. For technical reasons dynamical evolu- tion is studied by replacing the Minkowski by the Euclidean Quantum chromodynamics (QCD) represents part of the metric thereby converting to a statistical systems and using Standard Model which describes the strong interactions. Monte Carlo methods to evaluate the partition function. The fundamental degrees of freedom are quarks – the mat- In parallel many analytical many-body techniques have ter fields, and gluons – the mediators of the strong force. been employed to identify effective degrees of freedom and Quarks and gluons are permanently confined into hadrons, numerous approximation schemes have been advanced to e.g. protons, neutrons and pions, to within distance scales describe the soft structure and interactions of hadrons, e.g. of the order of 1fm = 10¡15m. Hadrons are bound by the constituent quark model, bag and topological soliton residual strong forces to form atomic nuclei. Thus QCD models, QCD sum rules, chiral lagrangians, etc. determines not only the quark-gluon dynamics at the sub- In this talk I will focus on the physics of soft gluonic ex- subatomic scale but also the interactions between nuclei at citations and their role in quark confinement. Gluons carry the subatomic scale and even the nuclear dynamics at a the strong force, and since on a hadronic scale the light u and macroscopic level, e.g. -

Neutron Stars
Chandra X-Ray Observatory X-Ray Astronomy Field Guide Neutron Stars Ordinary matter, or the stuff we and everything around us is made of, consists largely of empty space. Even a rock is mostly empty space. This is because matter is made of atoms. An atom is a cloud of electrons orbiting around a nucleus composed of protons and neutrons. The nucleus contains more than 99.9 percent of the mass of an atom, yet it has a diameter of only 1/100,000 that of the electron cloud. The electrons themselves take up little space, but the pattern of their orbit defines the size of the atom, which is therefore 99.9999999999999% Chandra Image of Vela Pulsar open space! (NASA/PSU/G.Pavlov et al. What we perceive as painfully solid when we bump against a rock is really a hurly-burly of electrons moving through empty space so fast that we can't see—or feel—the emptiness. What would matter look like if it weren't empty, if we could crush the electron cloud down to the size of the nucleus? Suppose we could generate a force strong enough to crush all the emptiness out of a rock roughly the size of a football stadium. The rock would be squeezed down to the size of a grain of sand and would still weigh 4 million tons! Such extreme forces occur in nature when the central part of a massive star collapses to form a neutron star. The atoms are crushed completely, and the electrons are jammed inside the protons to form a star composed almost entirely of neutrons. -

The Five Common Particles
The Five Common Particles The world around you consists of only three particles: protons, neutrons, and electrons. Protons and neutrons form the nuclei of atoms, and electrons glue everything together and create chemicals and materials. Along with the photon and the neutrino, these particles are essentially the only ones that exist in our solar system, because all the other subatomic particles have half-lives of typically 10-9 second or less, and vanish almost the instant they are created by nuclear reactions in the Sun, etc. Particles interact via the four fundamental forces of nature. Some basic properties of these forces are summarized below. (Other aspects of the fundamental forces are also discussed in the Summary of Particle Physics document on this web site.) Force Range Common Particles It Affects Conserved Quantity gravity infinite neutron, proton, electron, neutrino, photon mass-energy electromagnetic infinite proton, electron, photon charge -14 strong nuclear force ≈ 10 m neutron, proton baryon number -15 weak nuclear force ≈ 10 m neutron, proton, electron, neutrino lepton number Every particle in nature has specific values of all four of the conserved quantities associated with each force. The values for the five common particles are: Particle Rest Mass1 Charge2 Baryon # Lepton # proton 938.3 MeV/c2 +1 e +1 0 neutron 939.6 MeV/c2 0 +1 0 electron 0.511 MeV/c2 -1 e 0 +1 neutrino ≈ 1 eV/c2 0 0 +1 photon 0 eV/c2 0 0 0 1) MeV = mega-electron-volt = 106 eV. It is customary in particle physics to measure the mass of a particle in terms of how much energy it would represent if it were converted via E = mc2. -

Fundamentals of Particle Physics
Fundamentals of Par0cle Physics Particle Physics Masterclass Emmanuel Olaiya 1 The Universe u The universe is 15 billion years old u Around 150 billion galaxies (150,000,000,000) u Each galaxy has around 300 billion stars (300,000,000,000) u 150 billion x 300 billion stars (that is a lot of stars!) u That is a huge amount of material u That is an unimaginable amount of particles u How do we even begin to understand all of matter? 2 How many elementary particles does it take to describe the matter around us? 3 We can describe the material around us using just 3 particles . 3 Matter Particles +2/3 U Point like elementary particles that protons and neutrons are made from. Quarks Hence we can construct all nuclei using these two particles -1/3 d -1 Electrons orbit the nuclei and are help to e form molecules. These are also point like elementary particles Leptons We can build the world around us with these 3 particles. But how do they interact. To understand their interactions we have to introduce forces! Force carriers g1 g2 g3 g4 g5 g6 g7 g8 The gluon, of which there are 8 is the force carrier for nuclear forces Consider 2 forces: nuclear forces, and electromagnetism The photon, ie light is the force carrier when experiencing forces such and electricity and magnetism γ SOME FAMILAR THE ATOM PARTICLES ≈10-10m electron (-) 0.511 MeV A Fundamental (“pointlike”) Particle THE NUCLEUS proton (+) 938.3 MeV neutron (0) 939.6 MeV E=mc2. Einstein’s equation tells us mass and energy are equivalent Wave/Particle Duality (Quantum Mechanics) Einstein E -
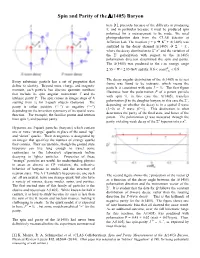
Spin and Parity of the Λ(1405) Baryon
Spin and Parity of the (1405) Baryon here [1], precisely because of the difficulty in producing it, and in particular because it must be produced spin polarized for a measurement to be made. We used photoproduction data from the CLAS detector at Jefferson Lab. The reaction + p K+ + (1405) was analyzed in the decay channel (1405) + + , where the decay distribution to + and the variation of the + polarization with respect to the (1405) polarization direction determined the spin and parity. The (1405) was produced in the c.m. energy range K 2.55 < W < 2.85 GeV and for 0.6 coscm.. 0.9 . The decay angular distribution of the (1405) in its rest Every subatomic particle has a set of properties that frame was found to be isotropic, which means the define its identity. Beyond mass, charge, and magnetic particle is consistent with spin J = ½. The first figure moment, each particle has discrete quantum numbers illustrates how the polarization P of a parent particle that include its spin angular momentum J and its with spin ½, in this case the (1405), transfers intrinsic parity P. The spin comes in integer steps of + starting from ½ for 3-quark objects (baryons). The polarization Q to the daughter baryon, in this case the , depending on whether the decay is in a spatial S wave parity is either positive (“+”) or negative (“”) (L=0) or P wave (L=1). This distinction is what depending on the inversion symmetry of its spatial wave determines the parity of the final state, and hence of the function. For example, the familiar proton and neutron parent. -

George De Hevesy in America
Journal of Nuclear Medicine, published on July 13, 2019 as doi:10.2967/jnumed.119.233254 George de Hevesy in America George de Hevesy was a Hungarian radiochemist who was awarded the Nobel Prize in Chemistry in 1943 for the discovery of the radiotracer principle (1). As the radiotracer principle is the foundation of all diagnostic and therapeutic nuclear medicine procedures, Hevesy is widely considered the father of nuclear medicine (1). Although it is well-known that he spent time at a number of European institutions, it is not widely known that he also spent six weeks at Cornell University in Ithaca, NY, in the fall of 1930 as that year’s Baker Lecturer in the Department of Chemistry (2-6). “[T]he Baker Lecturer gave two formal presentations per week, to a large and diverse audience and provided an informal seminar weekly for students and faculty members interested in the subject. The lecturer had an office in Baker Laboratory and was available to faculty and students for further discussion.” (7) There is also evidence that, “…Hevesy visited Harvard [University, Cambridge, MA] as a Baker Lecturer at Cornell in 1930…” (8). Neither of the authors of this Note/Letter was aware of Hevesy’s association with Cornell University despite our longstanding ties to Cornell until one of us (WCK) noticed the association in Hevesy’s biographical page on the official Nobel website (6). WCK obtained both his undergraduate degree and medical degree from Cornell in Ithaca and New York City, respectively, and spent his career in nuclear medicine. JRO did his nuclear medicine training at Columbia University and has subsequently been a faculty member of Weill Cornell Medical College for the last eleven years (with a brief tenure at Memorial Sloan Kettering Cancer Center (affiliated with Cornell)), and is now the program director of the Nuclear Medicine residency and Chief of the Molecular Imaging and Therapeutics Section. -

1 Standard Model: Successes and Problems
Searching for new particles at the Large Hadron Collider James Hirschauer (Fermi National Accelerator Laboratory) Sambamurti Memorial Lecture : August 7, 2017 Our current theory of the most fundamental laws of physics, known as the standard model (SM), works very well to explain many aspects of nature. Most recently, the Higgs boson, predicted to exist in the late 1960s, was discovered by the CMS and ATLAS collaborations at the Large Hadron Collider at CERN in 2012 [1] marking the first observation of the full spectrum of predicted SM particles. Despite the great success of this theory, there are several aspects of nature for which the SM description is completely lacking or unsatisfactory, including the identity of the astronomically observed dark matter and the mass of newly discovered Higgs boson. These and other apparent limitations of the SM motivate the search for new phenomena beyond the SM either directly at the LHC or indirectly with lower energy, high precision experiments. In these proceedings, the successes and some of the shortcomings of the SM are described, followed by a description of the methods and status of the search for new phenomena at the LHC, with some focus on supersymmetry (SUSY) [2], a specific theory of physics beyond the standard model (BSM). 1 Standard model: successes and problems The standard model of particle physics describes the interactions of fundamental matter particles (quarks and leptons) via the fundamental forces (mediated by the force carrying particles: the photon, gluon, and weak bosons). The Higgs boson, also a fundamental SM particle, plays a central role in the mechanism that determines the masses of the photon and weak bosons, as well as the rest of the standard model particles. -

The Subatomic Particle Mass Spectrum
The Subatomic Particle Mass Spectrum Robert L. Oldershaw 12 Emily Lane Amherst, MA 01002 USA [email protected] Key Words: Subatomic Particles; Particle Mass Spectrum; General Relativity; Kerr Metric; Discrete Self-Similarity; Discrete Scale Relativity 1 Abstract: Representative members of the subatomic particle mass spectrum in the 100 MeV to 7,000 MeV range are retrodicted to a first approximation using the Kerr solution of General Relativity. The particle masses appear to form a restricted set of quantized values of a Kerr- based angular momentum-mass relation: M = n1/2 M, where values of n are a set of discrete integers and M is a revised Planck mass. A fractal paradigm manifesting global discrete self- similarity is critical to a proper determination of M, which differs from the conventional Planck mass by a factor of roughly 1019. This exceedingly simple and generic mass equation retrodicts the masses of a representative set of 27 well-known particles with an average relative error of 1.6%. A more rigorous mass formula, which includes the total spin angular momentum rule of Quantum Mechanics, the canonical spin values of the particles, and the dimensionless rotational parameter of the Kerr angular momentum-mass relation, is able to retrodict the masses of the 8 dominant baryons in the 900 MeV to 1700 MeV range at the < 99.7% > level. 2 “There remains one especially unsatisfactory feature [of the Standard Model of particle physics]: the observed masses of the particles, m. There is no theory that adequately explains these numbers. We use the numbers in all our theories, but we do not understand them – what they are, or where they come from. -

7. Gamma and X-Ray Interactions in Matter
Photon interactions in matter Gamma- and X-Ray • Compton effect • Photoelectric effect Interactions in Matter • Pair production • Rayleigh (coherent) scattering Chapter 7 • Photonuclear interactions F.A. Attix, Introduction to Radiological Kinematics Physics and Radiation Dosimetry Interaction cross sections Energy-transfer cross sections Mass attenuation coefficients 1 2 Compton interaction A.H. Compton • Inelastic photon scattering by an electron • Arthur Holly Compton (September 10, 1892 – March 15, 1962) • Main assumption: the electron struck by the • Received Nobel prize in physics 1927 for incoming photon is unbound and stationary his discovery of the Compton effect – The largest contribution from binding is under • Was a key figure in the Manhattan Project, condition of high Z, low energy and creation of first nuclear reactor, which went critical in December 1942 – Under these conditions photoelectric effect is dominant Born and buried in • Consider two aspects: kinematics and cross Wooster, OH http://en.wikipedia.org/wiki/Arthur_Compton sections http://www.findagrave.com/cgi-bin/fg.cgi?page=gr&GRid=22551 3 4 Compton interaction: Kinematics Compton interaction: Kinematics • An earlier theory of -ray scattering by Thomson, based on observations only at low energies, predicted that the scattered photon should always have the same energy as the incident one, regardless of h or • The failure of the Thomson theory to describe high-energy photon scattering necessitated the • Inelastic collision • After the collision the electron departs -

TEK 8.5C: Periodic Table
Name: Teacher: Pd. Date: TEK 8.5C: Periodic Table TEK 8.5C: Interpret the arrangement of the Periodic Table, including groups and periods, to explain how properties are used to classify elements. Elements and the Periodic Table An element is a substance that cannot be separated into simpler substances by physical or chemical means. An element is already in its simplest form. The smallest piece of an element that still has the properties of that element is called an atom. An element is a pure substance, containing only one kind of atom. The Periodic Table of Elements is a list of all the elements that have been discovered and named, with each element listed in its own element square. Elements are represented on the Periodic Table by a one or two letter symbol, and its name, atomic number and atomic mass. The Periodic Table & Atomic Structure The elements are listed on the Periodic Table in atomic number order, starting at the upper left corner and then moving from the left to right and top to bottom, just as the words of a paragraph are read. The element’s atomic number is based on the number of protons in each atom of that element. In electrically neutral atoms, the atomic number also represents the number of electrons in each atom of that element. For example, the atomic number for neon (Ne) is 10, which means that each atom of neon has 10 protons and 10 electrons. Magnesium (Mg) has an atomic number of 12, which means it has 12 protons and 12 electrons. -

A Brief History of Nuclear Astrophysics
A BRIEF HISTORY OF NUCLEAR ASTROPHYSICS PART I THE ENERGY OF THE SUN AND STARS Nikos Prantzos Institut d’Astrophysique de Paris Stellar Origin of Energy the Elements Nuclear Astrophysics Astronomy Nuclear Physics Thermodynamics: the energy of the Sun and the age of the Earth 1847 : Robert Julius von Mayer Sun heated by fall of meteors 1854 : Hermann von Helmholtz Gravitational energy of Kant’s contracting protosolar nebula of gas and dust turns into kinetic energy Timescale ~ EGrav/LSun ~ 30 My 1850s : William Thompson (Lord Kelvin) Sun heated at formation from meteorite fall, now « an incadescent liquid mass » cooling Age 10 – 100 My 1859: Charles Darwin Origin of species : Rate of erosion of the Weald valley is 1 inch/century or 22 miles wild (X 1100 feet high) in 300 My Such large Earth ages also required by geologists, like Charles Lyell A gaseous, contracting and heating Sun 푀⊙ Mean solar density : ~1.35 g/cc Sun liquid Incompressible = 4 3 푅 3 ⊙ 1870s: J. Homer Lane ; 1880s :August Ritter : Sun gaseous Compressible As it shrinks, it releases gravitational energy AND it gets hotter Earth Mayer – Kelvin - Helmholtz Helmholtz - Ritter A gaseous, contracting and heating Sun 푀⊙ Mean solar density : ~1.35 g/cc Sun liquid Incompressible = 4 3 푅 3 ⊙ 1870s: J. Homer Lane ; 1880s :August Ritter : Sun gaseous Compressible As it shrinks, it releases gravitational energy AND it gets hotter Earth Mayer – Kelvin - Helmholtz Helmholtz - Ritter A gaseous, contracting and heating Sun 푀⊙ Mean solar density : ~1.35 g/cc Sun liquid Incompressible = 4 3 푅 3 ⊙ 1870s: J. -

The Avogadro Constant to Be Equal to Exactly 6.02214X×10 23 When It Is Expressed in the Unit Mol −1
[august, 2011] redefinition of the mole and the new system of units zoltan mester It is as easy to count atomies as to resolve the propositions of a lover.. As You Like It William Shakespeare 1564-1616 Argentina, Austria-Hungary, Belgium, Brazil, Denmark, France, German Empire, Italy, Peru, Portugal, Russia, Spain, Sweden and Norway, Switzerland, Ottoman Empire, United States and Venezuela 1875 -May 20 1875, BIPM, CGPM and the CIPM was established, and a three- dimensional mechanical unit system was setup with the base units metre, kilogram, and second. -1901 Giorgi showed that it is possible to combine the mechanical units of this metre–kilogram–second system with the practical electric units to form a single coherent four-dimensional system -In 1921 Consultative Committee for Electricity (CCE, now CCEM) -by the 7th CGPM in 1927. The CCE to proposed, in 1939, the adoption of a four-dimensional system based on the metre, kilogram, second, and ampere, the MKSA system, a proposal approved by the ClPM in 1946. -In 1954, the 10th CGPM, the introduction of the ampere, the kelvin and the candela as base units -in 1960, 11th CGPM gave the name International System of Units, with the abbreviation SI. -in 1970, the 14 th CGMP introduced mole as a unit of amount of substance to the SI 1960 Dalton publishes first set of atomic weights and symbols in 1805 . John Dalton(1766-1844) Dalton publishes first set of atomic weights and symbols in 1805 . John Dalton(1766-1844) Much improved atomic weight estimates, oxygen = 100 . Jöns Jacob Berzelius (1779–1848) Further improved atomic weight estimates .