Integrating California Populations of Fritillaria Gentneri Into the 2003
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Summary of Offerings in the PBS Bulb Exchange, Dec 2012- Nov 2019
Summary of offerings in the PBS Bulb Exchange, Dec 2012- Nov 2019 3841 Number of items in BX 301 thru BX 463 1815 Number of unique text strings used as taxa 990 Taxa offered as bulbs 1056 Taxa offered as seeds 308 Number of genera This does not include the SXs. Top 20 Most Oft Listed: BULBS Times listed SEEDS Times listed Oxalis obtusa 53 Zephyranthes primulina 20 Oxalis flava 36 Rhodophiala bifida 14 Oxalis hirta 25 Habranthus tubispathus 13 Oxalis bowiei 22 Moraea villosa 13 Ferraria crispa 20 Veltheimia bracteata 13 Oxalis sp. 20 Clivia miniata 12 Oxalis purpurea 18 Zephyranthes drummondii 12 Lachenalia mutabilis 17 Zephyranthes reginae 11 Moraea sp. 17 Amaryllis belladonna 10 Amaryllis belladonna 14 Calochortus venustus 10 Oxalis luteola 14 Zephyranthes fosteri 10 Albuca sp. 13 Calochortus luteus 9 Moraea villosa 13 Crinum bulbispermum 9 Oxalis caprina 13 Habranthus robustus 9 Oxalis imbricata 12 Haemanthus albiflos 9 Oxalis namaquana 12 Nerine bowdenii 9 Oxalis engleriana 11 Cyclamen graecum 8 Oxalis melanosticta 'Ken Aslet'11 Fritillaria affinis 8 Moraea ciliata 10 Habranthus brachyandrus 8 Oxalis commutata 10 Zephyranthes 'Pink Beauty' 8 Summary of offerings in the PBS Bulb Exchange, Dec 2012- Nov 2019 Most taxa specify to species level. 34 taxa were listed as Genus sp. for bulbs 23 taxa were listed as Genus sp. for seeds 141 taxa were listed with quoted 'Variety' Top 20 Most often listed Genera BULBS SEEDS Genus N items BXs Genus N items BXs Oxalis 450 64 Zephyranthes 202 35 Lachenalia 125 47 Calochortus 94 15 Moraea 99 31 Moraea -

Survey for Special-Status Vascular Plant Species
SURVEY FOR SPECIAL-STATUS VASCULAR PLANT SPECIES For the proposed Eagle Canyon Fish Passage Project Tehama and Shasta Counties, California Prepared for: Tehama Environmental Solutions 910 Main Street, Suite D Red Bluff, California 96080 Prepared by: Dittes & Guardino Consulting P.O. Box 6 Los Molinos, California 96055 (530) 384-1774 [email protected] Eagle Canyon Fish Passage Improvement Project - Botany Report Sept. 12, 2018 Prepared by: Dittes & Guardino Consulting 1 SURVEY FOR SPECIAL-STATUS VASCULAR PLANT SPECIES Eagle Canyon Fish Passage Project Shasta & Tehama Counties, California T30N, R1W, SE 1/4 Sec. 25, SE1/4 Sec. 24, NE ¼ Sec. 36 of the Shingletown 7.5’ USGS Topographic Quadrangle TABLE OF CONTENTS I. Executive Summary ................................................................................................................................................. 4 II. Introduction ............................................................................................................................................................ 4 III. Project Description ............................................................................................................................................... 4 IV. Location .................................................................................................................................................................. 5 V. Methods .................................................................................................................................................................. -

Analysis of the Giant Genomes of Fritillaria (Liliaceae) Indicates That a Lack of DNA Removal Characterizes Extreme Expansions in Genome Size
CORE Metadata, citation and similar papers at core.ac.uk Provided by Queen Mary Research Online Analysis of the giant genomes of Fritillaria (Liliaceae) indicates that a lack of DNA removal characterizes extreme expansions in genome size. Kelly, LJ; Renny-Byfield, S; Pellicer, J; Macas, J; Novák, P; Neumann, P; Lysak, MA; Day, PD; Berger, M; Fay, MF; Nichols, RA; Leitch, AR; Leitch, IJ © 2015 The Authors. CC-BY For additional information about this publication click this link. http://qmro.qmul.ac.uk/jspui/handle/123456789/8496 Information about this research object was correct at the time of download; we occasionally make corrections to records, please therefore check the published record when citing. For more information contact [email protected] Research Analysis of the giant genomes of Fritillaria (Liliaceae) indicates that a lack of DNA removal characterizes extreme expansions in genome size Laura J. Kelly1,2, Simon Renny-Byfield1,3, Jaume Pellicer2,Jirı Macas4, Petr Novak4, Pavel Neumann4, Martin A. Lysak5, Peter D. Day1,2, Madeleine Berger2,6,7, Michael F. Fay2, Richard A. Nichols1, Andrew R. Leitch1 and Ilia J. Leitch2 1School of Biological and Chemical Sciences, Queen Mary University of London, London, E1 4NS, UK; 2Jodrell Laboratory, Royal Botanic Gardens, Kew, Richmond, TW9 3DS, UK; 3 4 Department of Plant Sciences, University of California Davis, Davis, CA 95616, USA; Biology Centre CAS, Institute of Plant Molecular Biology, CZ-37005, Ceske Budejovice, Czech Republic; 5Plant Cytogenomics Research Group, CEITEC – Central European Institute of Technology, Masaryk University, Kamenice 5, CZ-62500, Brno, Czech Republic; 6School of Biological and Biomedical Sciences, Durham University, South Road, Durham DH1 3LE, UK; 7Rothamsted Research, West Common, Harpenden, Hertfordshire, AL5 2JQ, UK Summary Authors for correspondence: Plants exhibit an extraordinary range of genome sizes, varying by > 2000-fold between the Laura J. -

Gentner™S Fritillary
U.S. Fish and Wildlife Service Draft Recovery Plan for Fritillaria gentneri (Gentner’s fritillary) Cover photo: Fritillaria gentneri (Gentner’s fritillary) by Brad Tong, Medford District, Bureau of Land Management, used with permission. DRAFT RECOVERY PLAN FOR Fritillaria gentneri (Gentner’s fritillary) Region 1 U.S. Fish and Wildlife Service Portland, Oregon Approved: XXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXXX Regional Director, Region 1, U.S. Fish and Wildlife Service Date: DISCLAIMER Recovery plans delineate reasonable actions that are believed to be required to recover and/or protect listed species. Plans published by the U.S. Fish and Wildlife Service are sometimes prepared with the assistance of recovery teams, contractors, State agencies, and other affected and interested parties. Objectives of this plan will be attained and any necessary funds made available subject to budgetary and other constraints affecting the parties involved, as well as the need to address other priorities. Recovery plans do not obligate other parties to undertake specific tasks and may not represent the views or the official positions or approval of any individuals or agencies involved in the plan formulation, other than the U.S. Fish and Wildlife Service. Recovery plans represent the official position of the U.S. Fish and Wildlife Service only after they have been signed by the Director or Regional Director as approved. Approved recovery plans are subject to modification as dictated by new findings, changes in species status, and the completion of recovery tasks. Literature citation of this document should read as follows: U.S. Fish and Wildlife Service. 2002. Draft recovery plan for Fritillaria gentneri (Gentner’s fritillary). -

Selected Wildflowers of the Modoc National Forest Selected Wildflowers of the Modoc National Forest
United States Department of Agriculture Selected Wildflowers Forest Service of the Modoc National Forest An introduction to the flora of the Modoc Plateau U.S. Forest Service, Pacific Southwest Region i Cover image: Spotted Mission-Bells (Fritillaria atropurpurea) ii Selected Wildflowers of the Modoc National Forest Selected Wildflowers of the Modoc National Forest Modoc National Forest, Pacific Southwest Region U.S. Forest Service, Pacific Southwest Region iii Introduction Dear Visitor, e in the Modoc National Forest Botany program thank you for your interest in Wour local flora. This booklet was prepared with funds from the Forest Service Celebrating Wildflowers program, whose goals are to serve our nation by introducing the American public to the aesthetic, recreational, biological, ecological, medicinal, and economic values of our native botanical resources. By becoming more thoroughly acquainted with local plants and their multiple values, we hope to consequently in- crease awareness and understanding of the Forest Service’s management undertakings regarding plants, including our rare plant conservation programs, invasive plant man- agement programs, native plant materials programs, and botanical research initiatives. This booklet is a trial booklet whose purpose, as part of the Celebrating Wildflowers program (as above explained), is to increase awareness of local plants. The Modoc NF Botany program earnestly welcomes your feedback; whether you found the book help- ful or not, if there were too many plants represented or too few, if the information was useful to you or if there is more useful information that could be added, or any other comments or concerns. Thank you. Forest J. R. Gauna Asst. -

DRAFT OAEC NATIVE PLANT LIST FERNS and FERN ALLIES
DRAFT OAEC NATIVE PLANT LIST FERNS and FERN ALLIES: Blechnaceae: Deer Fern Family Giant Chain Fern Woodwardia fimbriata Dennstaedtiaceae: Bracken Fern Bracken Pteridium aquilinum Dryopteridaceae: Wood Fern Family Lady Fern Athyrium filix-femina Wood Fern Dryopteris argutanitum Western Sword Fern Polystichum muitum Polypodiaceae: Polypody Family California Polypody Polypodium californicum Pteridaceae: Brake Family California Maiden-Hair Adiantum jordanii Coffee Fern Pellaea andromedifolia Goldback Fern Pentagramma triangularis Isotaceae: Quillwort Family Isoetes sp? Nuttallii? Selaginellaceae: Spike-Moss Family Selaginella bigelovii GYMNOPSPERMS Pinaceae: Pine Family Douglas-Fir Psuedotsuga menziesii Taxodiaceae: Bald Cypress Family Redwood Sequoia sempervirens ANGIOSPERMS: DICOTS Aceraceae: Maple Family Big-Leaf Maple Acer macrophyllum Box Elder Acer negundo Anacardiaceae: Sumac Family Western Poison Oak Toxicodendron diversilobum Apiaceae: Carrot Family Lomatium( utriculatum) or (carulifolium)? Pepper Grass Perideridia kelloggii Yampah Perideridia gairdneri Sanicula sp? Sweet Cicely Osmorhiza chilensis Unidentified in forest at barn/deer fence gate Angelica Angelica tomentosa Apocynaceae: Dogbane or Indian Hemp Family Apocynum cannabinum Aristolochiaceae Dutchman’s Pipe, Pipevine Aristolochia californica Wild Ginger Asarum caudatum Asteraceae: Sunflower Family Grand Mountain Dandelion Agoseris grandiflora Broad-leaved Aster Aster radulinus Coyote Brush Baccharis pilularis Pearly Everlasting Anaphalis margaritacea Woodland Tarweed Madia -
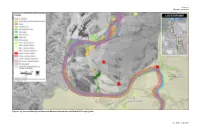
Botanical Resources and Wetlands Technical Report
Chapter 1 Affected Environment Figure 1-3g. Sensitive Biological Resources Between Shasta Dam and Red Bluff Pumping Plant 1-45 Draft – June 2013 Shasta Lake Water Resources Investigation Biological Resources Appendix – Botanical Resources and Wetlands Technical Report This page left blank intentionally. 1-46 Draft – June 2013 Chapter 1 Affected Environment Figure 1-3h. Sensitive Biological Resources Between Shasta Dam and Red Bluff Pumping Plant 1-47 Draft – June 2013 Shasta Lake Water Resources Investigation Biological Resources Appendix – Botanical Resources and Wetlands Technical Report This page left blank intentionally. 1-48 Draft – June 2013 Chapter 1 Affected Environment Figure 1-3i. Sensitive Biological Resources Between Shasta Dam and Red Bluff Pumping Plant 1-49 Draft – June 2013 Shasta Lake Water Resources Investigation Biological Resources Appendix – Botanical Resources and Wetlands Technical Report This page left blank intentionally. 1-50 Draft – June 2013 Chapter 1 Affected Environment Figure 1-3j. Sensitive Biological Resources Between Shasta Dam and Red Bluff Pumping Plant 1-51 Draft – June 2013 Shasta Lake Water Resources Investigation Biological Resources Appendix – Botanical Resources and Wetlands Technical Report This page left blank intentionally. 1-52 Draft – June 2013 Chapter 1 Affected Environment 1 Valley Oak Woodland This habitat type consists of an open savanna of 2 valley oak (Quercus lobata) trees and an annual grassland understory. Valley 3 oak is typically the only tree species present and shrubs are generally absent 4 except for occasional poison oak. Canopy cover rarely exceeds 30–40 percent in 5 valley oak woodland. This community occupies the highest portions of the 6 floodplain terrace where flooding is infrequent and shallow. -

Sierra Azul Wildflower Guide
WILDFLOWER SURVEY 100 most common species 1 2/25/2020 COMMON WILDFLOWER GUIDE 2019 This common wildflower guide is for use during the annual wildflower survey at Sierra Azul Preserve. Featured are the 100 most common species seen during the wildflower surveys and only includes flowering species. Commonness is based on previous surveys during April for species seen every year and at most areas around Sierra Azul OSP. The guide is a simple color photograph guide with two selected features showcasing the species—usually flower and whole plant or leaf. The plants in this guide are listed by Color. Information provided includes the Latin name, common name, family, and Habit, CNPS Inventory of Rare and Endangered Plants rank or CAL-IPC invasive species rating. Latin names are current with the Jepson Manual: Vascular Plants of California, 2012. This guide was compiled by Cleopatra Tuday for Midpen. Images are used under creative commons licenses or used with permission from the photographer. All image rights belong to respective owners. Taking Good Photos for ID: How to use this guide: Take pictures of: Flower top and side; Leaves top and bottom; Stem or branches; Whole plant. llama squash Cucurbitus llamadensis LLAMADACEAE Latin name 4.2 Shrub Common name CNPS rare plant rank or native status Family name Typical bisexual flower stigma pistil style stamen anther Leaf placement filament petal (corolla) sepal (calyx) alternate opposite whorled pedicel receptacle Monocots radial symmetry Parts in 3’s, parallel veins Typical composite flower of the Liliy, orchid, iris, grass Asteraceae (sunflower) family 3 ray flowers disk flowers Dicots Parts in 4’s or 5’s, lattice veins 4 Sunflowers, primrose, pea, mustard, mint, violets phyllaries bilateral symmetry peduncle © 2017 Cleopatra Tuday 2 2/25/2020 BLUE/PURPLE ©2013 Jeb Bjerke ©2013 Keir Morse ©2014 Philip Bouchard ©2010 Scott Loarie Jim brush Ceanothus oliganthus Blue blossom Ceanothus thyrsiflorus RHAMNACEAE Shrub RHAMNACEAE Shrub ©2003 Barry Breckling © 2009 Keir Morse Many-stemmed gilia Gilia achilleifolia ssp. -

W a Sh in G to N Na Tu Ra L H Er Itag E Pr Og Ra M
PROGRAM HERITAGE NATURAL Conservation Status Ranks of Washington’s Ecological Systems Prepared for Washington Dept. of Fish and WASHINGTON Wildlife Prepared by F. Joseph Rocchio and Rex. C. Crawford August 04, 2015 Natural Heritage Report 2015-03 Conservation Status Ranks for Washington’s Ecological Systems Washington Natural Heritage Program Report Number: 2015-03 August 04, 2015 Prepared by: F. Joseph Rocchio and Rex C. Crawford Washington Natural Heritage Program Washington Department of Natural Resources Olympia, Washington 98504-7014 .ON THE COVER: (clockwise from top left) Crab Creek (Inter-Mountain Basins Big Sagebrush Steppe and Columbia Basin Foothill Riparian Woodland and Shrubland Ecological Systems); Ebey’s Landing Bluff Trail (North Pacific Herbaceous Bald and Bluff Ecological System and Temperate Pacific Tidal Salt and Brackish Marsh Ecological Systems); and Judy’s Tamarack Park (Northern Rocky Mountain Western Larch Savanna). Photographs by: Joe Rocchio Table of Contents Page Table of Contents ............................................................................................................................ ii Tables ............................................................................................................................................. iii Introduction ..................................................................................................................................... 4 Methods.......................................................................................................................................... -

Rock Garden Quarterly
ROCK GARDEN QUARTERLY VOLUME 55 NUMBER 2 SPRING 1997 COVER: Tulipa vvedevenskyi by Dick Van Reyper All Material Copyright © 1997 North American Rock Garden Society Printed by AgPress, 1531 Yuma Street, Manhattan, Kansas 66502 ROCK GARDEN QUARTERLY BULLETIN OF THE NORTH AMERICAN ROCK GARDEN SOCIETY VOLUME 55 NUMBER 2 SPRING 1997 FEATURES Life with Bulbs in an Oregon Garden, by Molly Grothaus 83 Nuts about Bulbs in a Minor Way, by Andrew Osyany 87 Some Spring Crocuses, by John Grimshaw 93 Arisaema bockii: An Attenuata Mystery, by Guy Gusman 101 Arisaemas in the 1990s: An Update on a Modern Fashion, by Jim McClements 105 Spider Lilies, Hardy Native Amaryllids, by Don Hackenberry 109 Specialty Bulbs in the Holland Industry, by Brent and Becky Heath 117 From California to a Holland Bulb Grower, by W.H. de Goede 120 Kniphofia Notes, by Panayoti Kelaidis 123 The Useful Bulb Frame, by Jane McGary 131 Trillium Tricks: How to Germinate a Recalcitrant Seed, by John F. Gyer 137 DEPARTMENTS Seed Exchange 146 Book Reviews 148 82 ROCK GARDEN QUARTERLY VOL. 55(2) LIFE WITH BULBS IN AN OREGON GARDEN by Molly Grothaus Our garden is on the slope of an and a recording thermometer, I began extinct volcano, with an unobstructed, to discover how large the variation in full frontal view of Mt. Hood. We see warmth and light can be in an acre the side of Mt. Hood facing Portland, and a half of garden. with its top-to-bottom 'H' of south tilt• These investigations led to an inter• ed ridges. -

SRGC BULB LOG DIARY---Pictures and Text © Ian Young
SRGC ----- Bulb Log Diary ----- Pictures and text © Ian Young BULB LOG 18……………………………….6th May 2009 Tulips and Ipheion I am very pleased with the way that the tulips are looking in the narrow bed at the base of a south facing wall. They seem to be settling in so well that I think I will plant more outside this summer. I have a number of pots of tulips in the Frit house, (see bulb log 16) where they are also growing well but the stems are at least twice the height of those outside. I think the plan will be to plant most of them out and just keep a bulb or two under glass as an insurance against outside losses. Narcissus abscissus Narcissus nevadensis Also growing well outside are these two Narcissus raised from SRGC seed many years ago and I think I have the correct names for them. Both are very elegant trumpet daffodils. As they do not seem to be dividing at any rate I am hoping for a good seed set to help me increase my stock. Erythronium and Pond Sadly the peak flowering of the Erythroniums is over for another year but there are still a number of late flowering forms and species with flowers on. Looking across the bed at the top of the garden where I have been letting the Erythroniums naturalise under the larger Rhododendrons I am hopeful of a better seed set than I had last year when the weather at flowering time was terrible resulting in virtually no seeds. It has not been that good every day this year but at least the temperature was moderately better and I am watching carefully for signs of the seed pods turning upright and fattening. -

Botany Biological Assessment/ Biological Evaluation
Botany Biological Assessment/ Biological Evaluation for the Lehigh Southwest Land Exchange Shasta-Trinity National Recreation Area Shasta-Trinity National Forest Shasta County, California December 2012 Prepared by: 3/13/13 Leslie Perry, Environmental Analyst/Biologist Date Reviewed by: Martin Lenz, Shasta Lake District Botanist Date Botany BA/BE Lehigh Southwest Land Exchange FINAL I. PROJECT DESCRIPTION The Shasta-Trinity National Forest (STNF) proposes to exchange lands with Lehigh Southwest Cement Company (Lehigh) and approve a non-significant amendment to the STNF Land and Resource Management Plan (U.S. Forest Service 1994). The lands to be exchanged include two Federal parcels managed by the Forest Service near the Gray Rocks quarry on the south side of Shasta Lake east of Interstate 5, encompassing approximately 62.56 acres, and one private parcel owned by Lehigh on the east side of Shasta Lake at the McCloud River arm, encompassing up to approximately 243.94 acres (specific acreage to be determined during land appraisal). Easements on Road 33N99 would also be exchanged as part of the land transfer to maintain access for each party across the lands. The lands and Road 33N99 are in the Shasta Unit of the Whiskeytown-Shasta-Trinity National Recreation Area on the STNF in Shasta County, California. The purpose of the exchange from private to Federal ownership is to consolidate National Forest ownership of lands in the Shasta Unit of the NRA and protect high quality plant and wildlife habitat along the McCloud River arm of Shasta Lake. A complete description of the purpose and need and alternatives can be found in the Environmental Assessment (see project record).