Plant List Olallie Mountain
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Central Composite Design for Optimizing the Biosynthesis Of
www.nature.com/scientificreports OPEN Central Composite Design for Optimizing the Biosynthesis of Silver Nanoparticles using Plantago major Extract and Investigating Antibacterial, Antifungal and Antioxidant Activity Ghazal Nikaeen1, Saeed Yousefnejad1 ✉ , Samane Rahmdel2, Fayezeh Samari3 & Saeideh Mahdavinia1 Central composite design (CCD) was applied to optimize the synthesis condition of silver nanoparticles (AgNPs) using the extract of Plantago major (P. major) seeds via a low cost and single-step process. The aqueous seed extract was applied as both reducing element and capping reagent for green production of AgNPs. Five empirical factors of synthesis including temperature (Temp), pH, volume of P. major extract (Vex), volume of AgNO3 solution (VAg) and synthesis time were used as independent variables of model and peak intensity of Surface Plasmon Resonance (SPR) originated from NPs as the dependent variable. The predicted optimal conditions was determined to be: Temp = 55 °C, pH = 9.9,Vex = 1.5 mL, VAg = 30 mL, time = 60 min. The characterization of the prepared AgNPs at these optimum conditions was conducted by Fourier transform infrared spectroscopy (FTIR), dynamic light scattering (DLS), transmission electron microscopy (TEM) and X-ray difraction (XRD) to determine the surface bio- functionalities. Bio-activity of these AgNPs against bacteria and fungi were evaluated based on its assay against Micrococcus luteus, Escherichia coli and Penicillium digitatum. Furthermore, antioxidant capacity of these NPs was checked using the ferric reducing antioxidant power (FRAP) assay. Nanotechnology is an important feld of modern research which has been the principal of various technologies and main innovations; and is expected to be the basis of many other outstanding innovations in future. -
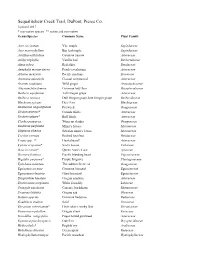
Sequalitchew Creek Trail Plant List
Sequalitchew Creek Trail, DuPont, Pierce Co. Updated 2017 * non-native species ** native and non-native Genus/Species Common Name Plant Family Acer circinatum Vine maple Sapindaceae Acer macrophyllum Big leaf maple Sapindaceae Achillea millefolium Common yarrow Asteraceae Achlys triphylla Vanilla leaf Berberidaceae Alnus rubra Red alder Betulaceae Anaphalis margaritacea Pearly everlasting Asteraceae Arbutus menziesii Pacific madrone Ericaceae Artemisia suksdorfii Coastal wormwood Asteraceae Asarum caudatum Wild ginger Aristolochiaceae Athyrium felix-femina Common lady fern Dryopteridaceae Berberis aquifolium Tall Oregon grape Asteraceae Berberis nervosa Dull Oregon grape, low Oregon grape Berberidaceae Blechnum spicant Deer fern Blechnaceae Chamerion angustifolium Fireweed Onagraceae Cirsium arvense* Canada thistle Asteraceae Cirsium vulgare* Bull thistle Asteraceae Clarkia purpurea Winecup clarkia Onagraceae Claytonia perfoliata Miner's lettuce Montiaceae Claytonia siberica Siberian miner's lettuce Montiaceae Corylus cornuta Beaked hazelnut Betulaceae Crepis spp. ?* Hawksbeard? Asteraceae Cytisus scoparius* Scot's broom Fabaceae Daucus carota* Queen Anne's Lace Apiaceae Dicentra formosa Pacific bleeding heart Papaveraceae Digitalis purpurea* Purple foxglove Plantaginaceae Epilobium minutum Threadstem fireweed Onagraceae Equisetum arvense Common horsetail Equisetaceae Equisetum telmateia Giant horsetail Equisetaceae Eriophyllum lanatum Oregon sunshine Asteraceae Erythronium oregonum White fawn lily Liliaceae Frangula purshiana Cascara, -

Fort Ord Natural Reserve Plant List
UCSC Fort Ord Natural Reserve Plants Below is the most recently updated plant list for UCSC Fort Ord Natural Reserve. * non-native taxon ? presence in question Listed Species Information: CNPS Listed - as designated by the California Rare Plant Ranks (formerly known as CNPS Lists). More information at http://www.cnps.org/cnps/rareplants/ranking.php Cal IPC Listed - an inventory that categorizes exotic and invasive plants as High, Moderate, or Limited, reflecting the level of each species' negative ecological impact in California. More information at http://www.cal-ipc.org More information about Federal and State threatened and endangered species listings can be found at https://www.fws.gov/endangered/ (US) and http://www.dfg.ca.gov/wildlife/nongame/ t_e_spp/ (CA). FAMILY NAME SCIENTIFIC NAME COMMON NAME LISTED Ferns AZOLLACEAE - Mosquito Fern American water fern, mosquito fern, Family Azolla filiculoides ? Mosquito fern, Pacific mosquitofern DENNSTAEDTIACEAE - Bracken Hairy brackenfern, Western bracken Family Pteridium aquilinum var. pubescens fern DRYOPTERIDACEAE - Shield or California wood fern, Coastal wood wood fern family Dryopteris arguta fern, Shield fern Common horsetail rush, Common horsetail, field horsetail, Field EQUISETACEAE - Horsetail Family Equisetum arvense horsetail Equisetum telmateia ssp. braunii Giant horse tail, Giant horsetail Pentagramma triangularis ssp. PTERIDACEAE - Brake Family triangularis Gold back fern Gymnosperms CUPRESSACEAE - Cypress Family Hesperocyparis macrocarpa Monterey cypress CNPS - 1B.2, Cal IPC -

Estrogen and Thyroid Hormone Receptor Activation by Medicinal Plants from Bahia, Brazil
medicines Article Estrogen and Thyroid Hormone Receptor Activation by Medicinal Plants from Bahia, Brazil Luã Tainã Costa Reis 1 ID , Magnus Régios Dias da Silva 2, Silvia Lima Costa 3, Eudes da Silva Velozo 4, Ronan Batista 5 ID and Suzana Telles da Cunha Lima 1,* ID 1 Laboratory of Bioprospection and Biotechnology (LaBBiotec), Institute of Biology, Federal University of Bahia (UFBA), Barão de Jeremoabo Street, 147-Ondina, Salvador, BA 40170-115, Brazil; [email protected] 2 Laboratory of Molecular and Translational Endocrinology, Department of Medicine, Federal University of São Paulo (UNIFESP), R. Sena Madureira, 1500-Vila Clementino, São Paulo, SP 04021-001, Brazil; [email protected] 3 Laboratory of Neurochemistry and Cell Biology, Department of Biofunction, Institute of Health Sciences, Federal University of Bahia (UFBA), Reitor Miguel Calmon Avenue, 1272-Canela, Salvador, BA 40231-300, Brazil; [email protected] 4 Laboratory of Research in Materia Medica, Department of Medicament, Faculty of Pharmacy, Federal University of Bahia (UFBA), Barão de Jeremoabo Street, 147-Ondina, Salvador, BA 40170-115, Brazil; [email protected] 5 Department of Organic Chemistry, Institute of Chemistry, Federal University of Bahia (UFBA), Barão de Jeremoabo Street, 147-Ondina, Salvador, BA 40170-115, Brazil; [email protected] * Correspondence: [email protected]; Tel.: +55-71-987-938-847 Received: 23 December 2017; Accepted: 11 January 2018; Published: 15 January 2018 Abstract: Background: A number of medicinal plants are traditionally used for metabolic disorders in Bahia state, Brazil. The aim of this study was to evaluate the estrogen receptor (ER) and thyroid receptor (TR) activation of crude extracts prepared from 20 plants. -

Ethnobotanical Survey of Medicinal Plants Used by the Natives of Umuahia, Abia State, Nigeria for the Management of Diabetes
IOSR Journal Of Pharmacy And Biological Sciences (IOSR-JPBS) e-ISSN:2278-3008, p-ISSN:2319-7676. Volume 14, Issue 5 Ser. I (Sep – Oct 2019), PP 05-37 www.Iosrjournals.Org Ethnobotanical Survey of Medicinal Plants Used By the Natives of Umuahia, Abia State, Nigeria for the Management of Diabetes Anowi Chinedu Fredrick1 , Uyanwa Ifeanyi Christian1 1 Department of Pharmacognosy and Traditional Medicine, Faculty of Pharmaceutical Sciences, Nnamdi Azikiwe University, Awka, Nigeria. Corresponding Author: Anowi Chinedu Fredrick Abstract: Diabetes has been regarded as one of the major health problems wrecking havoc on the people especially the geriatrics. In Umuahia, diabetes is regarded as a serious health problems with high rate of mortality, morbidity and with serious health consequences. Currently plants are used by the natives to treat this disease. Hence the need for this study to ascertain medicinal plants with high cure rate but little side effects as synthetic antidiabetic drugs have been known to be associated with various serious and deleterious side effects. This is therefore a field trip conducted in Umuahia, Nigeria, to determine the various medicinal plants used by the natives in the management of diabetes. Dialogue in the form of semi-structured interview was conducted with the traditional healers (TH). Some of whom were met many times depending on the amount of information available at any given time and to check the already collected information. Information regarding the plants used in the management /treatment of diabetes were collected, the socio-political data of the THs, formulation of remedies, and the symptoms and other ways the THs use to diagnose diabetes. -

Plant List Lomatium Mohavense Mojave Parsley 3 3 Lomatium Nevadense Nevada Parsley 3 Var
Scientific Name Common Name Fossil Falls Alabama Hills Mazourka Canyon Div. & Oak Creeks White Mountains Fish Slough Rock Creek McGee Creek Parker Bench East Mono Basin Tioga Pass Bodie Hills Cicuta douglasii poison parsnip 3 3 3 Cymopterus cinerarius alpine cymopterus 3 Cymopterus terebinthinus var. terebinth pteryxia 3 3 petraeus Ligusticum grayi Gray’s lovage 3 Lomatium dissectum fern-leaf 3 3 3 3 var. multifidum lomatium Lomatium foeniculaceum ssp. desert biscuitroot 3 fimbriatum Plant List Lomatium mohavense Mojave parsley 3 3 Lomatium nevadense Nevada parsley 3 var. nevadense Lomatium rigidum prickly parsley 3 Taxonomy and nomenclature in this species list are based on Lomatium torreyi Sierra biscuitroot 3 western sweet- the Jepson Manual Online as of February 2011. Changes in Osmorhiza occidentalis 3 3 ADOXACEAE–ASTERACEAE cicely taxonomy and nomenclature are ongoing. Some site lists are Perideridia bolanderi Bolander’s 3 3 more complete than others; all of them should be considered a ssp. bolanderi yampah Lemmon’s work in progress. Species not native to California are designated Perideridia lemmonii 3 yampah with an asterisk (*). Please visit the Inyo National Forest and Perideridia parishii ssp. Parish’s yampah 3 3 Bureau of Land Management Bishop Resource Area websites latifolia for periodic updates. Podistera nevadensis Sierra podistera 3 Sphenosciadium ranger’s buttons 3 3 3 3 3 capitellatum APOCYNACEAE Dogbane Apocynum spreading 3 3 androsaemifolium dogbane Scientific Name Common Name Fossil Falls Alabama Hills Mazourka Canyon Div. & Oak Creeks White Mountains Fish Slough Rock Creek McGee Creek Parker Bench East Mono Basin Tioga Pass Bodie Hills Apocynum cannabinum hemp 3 3 ADOXACEAE Muskroot Humboldt Asclepias cryptoceras 3 Sambucus nigra ssp. -

Proceedings: Shrubland Dynamics -- Fire and Water
Proceedings: Shrubland Dynamics—Fire and Water Lubbock, TX, August 10-12, 2004 United States Department of Agriculture Forest Service Rocky Mountain Research Station Proceedings RMRS-P-47 July 2007 Sosebee, Ronald E.; Wester, David B.; Britton, Carlton M.; McArthur, E. Durant; Kitchen, Stanley G., comps. 2007. Proceedings: Shrubland dynamics—fire and water; 2004 August 10-12; Lubbock, TX. Proceedings RMRS-P-47. Fort Collins, CO: U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station. 173 p. Abstract The 26 papers in these proceedings are divided into five sections. The first two sections are an introduction and a plenary session that introduce the principles and role of the shrub life-form in the High Plains, including the changing dynamics of shrublands and grasslands during the last four plus centuries. The remaining three sections are devoted to: fire, both prescribed fire and wildfire, in shrublands and grassland-shrubland interfac- es; water and ecophysiology shrubland ecosystems; and the ecology and population biology of several shrub species. Keywords: wildland shrubs, fire, water, ecophysiology, ecology The use of trade or firm names in this publication is for reader information and does not imply endorsement by the U.S. Department of Agriculture or any product or service. Publisher’s note: Papers in this report were reviewed by the compilers. Rocky Mountain Research Station Publishing Services reviewed papers for format and style. Authors are responsible for content. You may order additional copies of this publication by sending your mailing information in label form through one of the following media. Please specify the publication title and series number. -

Washington Flora Checklist a Checklist of the Vascular Plants of Washington State Hosted by the University of Washington Herbarium
Washington Flora Checklist A checklist of the Vascular Plants of Washington State Hosted by the University of Washington Herbarium The Washington Flora Checklist aims to be a complete list of the native and naturalized vascular plants of Washington State, with current classifications, nomenclature and synonymy. The checklist currently contains 3,929 terminal taxa (species, subspecies, and varieties). Taxa included in the checklist: * Native taxa whether extant, extirpated, or extinct. * Exotic taxa that are naturalized, escaped from cultivation, or persisting wild. * Waifs (e.g., ballast plants, escaped crop plants) and other scarcely collected exotics. * Interspecific hybrids that are frequent or self-maintaining. * Some unnamed taxa in the process of being described. Family classifications follow APG IV for angiosperms, PPG I (J. Syst. Evol. 54:563?603. 2016.) for pteridophytes, and Christenhusz et al. (Phytotaxa 19:55?70. 2011.) for gymnosperms, with a few exceptions. Nomenclature and synonymy at the rank of genus and below follows the 2nd Edition of the Flora of the Pacific Northwest except where superceded by new information. Accepted names are indicated with blue font; synonyms with black font. Native species and infraspecies are marked with boldface font. Please note: This is a working checklist, continuously updated. Use it at your discretion. Created from the Washington Flora Checklist Database on September 17th, 2018 at 9:47pm PST. Available online at http://biology.burke.washington.edu/waflora/checklist.php Comments and questions should be addressed to the checklist administrators: David Giblin ([email protected]) Peter Zika ([email protected]) Suggested citation: Weinmann, F., P.F. Zika, D.E. Giblin, B. -

Genetic Variation, Biochemical Contents and Wound Healing Activity of Plantago Major
Genetic Variation, Biochemical Contents and Wound Healing Activity of Plantago major Muhammad Zubair Faculty of Landscape Planning, Horticulture and Agricultural Sciences Department of Plant Breeding and Biotechnology Balsgård Doctoral Thesis Swedish University of Agricultural Sciences Alnarp 2012 Acta Universitatis Agriculturae Sueciae: doctoral thesis 2012:20 Cover: RAPD banding patterns on agarose gel, structure of plantamajoside, cells in a scratch assay, Plantago major plant, cells showing NF-kB presence in nuclear and cytoplasm, pig ear wound biopsy. (photos: J. M. Brandner; K. Rumpunen; M. Zubair) ISSN 1652-6880 ISBN 978-91-576-7656-6 © 2012 Muhammad Zubair, Alnarp Print: SLU Service/Repro, Alnarp 2012 Genetic Variation, Biochemical Contents and Wound Healing Activity of Plantago major Abstract Plantago major L. (greater plantain, common plantain) has been used as a wound healing remedy in different parts of the world for centuries. Different bioactive compounds have been proposed to contribute to the wound healing properties of this plant. The present study was undertaken to investigate the impact of some genetic and environmental factors on the wound healing activity of common plantain. Seeds of P. major were collected from five populations in different parts of Sweden, and were germinated and grown in a greenhouse. As expected for an inbreeding species, RAPD analyses demonstrated considerable between- population variation but very sparse within-population and within- subpopulation variation. Six major phenolic compounds were encountered in samples of P. major, four of which were identified for the first time in this thesis; PLMA 1–PLMA 4. Between-population and sub-population differences in the contents of these chemical compounds showed no correlation with RAPD-based estimates of genetic relatedness. -

Medlcinal PLANTS of JAMAICA. PARTS 1 &
MEDlCINAL PLANTS OF JAMAICA. PARTS 1 & 11. By G. F. Asprey, M.Sc., Ph.D. (B'ham.), Professor of Botany, U.C.W.l. and Phyllis Thornton, B.Sc. (Liverpool), Botanist Vomiting Sickness Survey. Attached to Botany Depart- ment, U.C.W.l. Reprinted from the West Indian Medical Journal. Vol. 2 No. 4. Vol. 3 No. 1. MEDICINAL PLANTS OF JAMAICA G. F. ASPREY AND PHYLLIS THORNTON PART I The use of local plants for medicinal remedies is a very prevalent practice in Jamaica. Among the poorer families, the morning meal frequently consists of nothing more than a cup of bush-tea prepared by steeping the leaves in hot water, with perhaps a small piece of bread or a little corn meal porridge. It is perhaps significant that the term breakfast is not used but 'taking' or ‘drinking' tea is substituted. Many of the plants used for treatment of colds and indigestion also provide the normal morning drinks. The large number of 'cold', 'fever' (which includes malaria) and 'indigestion' remedies is of some interest as providing a guide to the frequency of these complaints. The claims made for some of the plants may occasionally be justified by their chemical constituents. Some of them are, or have been, in the pharmacopoeias. On the other hand, in many cases the claims either have little justification or remain to be substantiated. Many of the doses used are of an unpleasant and even drastic nature. This may account for their popularity in view of the general impression that medicine must be unpleasant to be efficacious. -

Plantago Media L.—Explored and Potential Applications of an Underutilized Plant
plants Review Plantago media L.—Explored and Potential Applications of an Underutilized Plant Radu Claudiu Fierascu 1,2, Irina Fierascu 1,3,* , Alina Ortan 3 and Alina Paunescu 4 1 National Institute for Research & Development in Chemistry and Petrochemistry—ICECHIM, 060021 Bucharest, Romania; fi[email protected] 2 Department of Science and Engineering of Oxide Materials and Nanomaterials, University “Politehnica” of Bucharest, 011061 Bucharest, Romania 3 University of Agronomic Sciences and Veterinary Medicine of Bucharest, 011464 Bucharest, Romania; [email protected] 4 Department of Natural Sciences, University of Pitesti, 1 Targu din Vale Str., Pitesti, 110040 Arges, Romania; [email protected] * Correspondence: [email protected] Abstract: The search of valuable natural compounds should be directed towards alternative vegetal resources, and to the re-discovery of underutilized plants. Belonging to the Plantaginaceae family, the hoary plantain (Plantago media L.) represents one of the lesser studied species from the Plantago genus. The literature study revealed the under-utilization of the hoary plantain, a surprising aspect, considering its widespread. If the composition of Plantago media L. is rather well established, its applications are not nearly studied as for other Plantago species. The goal of the present paper is to summarize the findings regarding the applications of P. media, and, having as starting point the applications of related species, to propose new emerging areas of research, such as the biomedical applications validation through in vivo assays, and the evaluation of its potential towards indus- trial applications (i.e., development of food or personal care products), pisciculture or zootechny, phytoremediation and other environmental protection applications, or in the nanotechnology area Citation: Fierascu, R.C.; Fierascu, I.; (materials phytosynthesis). -

Woodard Bay Natural Resources Conservation Area Vascular Plant List
Woodard Bay Natural Resources Conservation Area Vascular Plant List Courtesy of DNR staff and the Washington Native Plant Society. Nomenclature follows Flora of the Pacific Northwest 2nd Edition (2018). * - Introduced Genus/Species Common Name Plant Family Abies grandis Grand fir Pinaceae Acer circinatum Vine maple Sapindaceae Acer macrophyllum Bigleaf maple Sapindaceae Achillea millefolium Common yarrow Asteraceae Achlys triphylla Vanilla leaf Berberidaceae Actaea rubra Baneberry Ranunculacae Adenocaulon bicolor Path finder, trail plant Asteraceae Adiantum aleuticum (A. pedantum) Northern maidenhair fern Pteridaceae Agrostis exarata Spike bentgrass Poaceae Aira caryophyllea* Silver hairgrass Poaceae Amelanchier alnifolia Western serviceberry, saskatoon Rosaceae Anaphalis margaritacea Pearly everlasting Asteraceae Anthemis cotula* Stinking chamomile, mayweed chamomile Asteraceae Aquilegia formosa Red columbine Ranunculaceae Arbutus menziesii Pacific madrone Ericaceae Arctostaphylos uva-ursi Kinnikinnick Ericaceae Arctium minus* Common burdock Asteraceae Arum italicum* Italian arum Araceae Asarum caudatum Wild ginger Aristolochiaceae Athyrium filix-femina Common lady fern, northern lady fern Athyriaceae Atriplex patula* Saltbush, spearscale Amaranthaceae Bellis perennis* English lawn daisy Asteraceae Mahonia aquifolium Tall Oregon grape Berberidaceae Mahonia nervosa Dull Oregon-grape, Low Oregon-grape Berberidaceae Struthiopteris spicant Deer fern Blechnaceae Brassica nigra* Black mustard Brassicaceae Bromus spp** Brome grasses Poaceae