Merkel Cell Carcinoma in Situ Case 2: Management and Outcome
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

View Open Access Histological Variants of Cutaneous Kaposi Sarcoma Wayne Grayson1 and Liron Pantanowitz*2
Diagnostic Pathology BioMed Central Review Open Access Histological variants of cutaneous Kaposi sarcoma Wayne Grayson1 and Liron Pantanowitz*2 Address: 1Histopathology Department, Ampath National Laboratory Support Services, Johannesburg, South Africa and 2Department of Pathology, Baystate Medical Center, Tufts University School of Medicine, Springfield, Massachusetts, USA Email: Wayne Grayson - [email protected]; Liron Pantanowitz* - [email protected] * Corresponding author Published: 25 July 2008 Received: 23 July 2008 Accepted: 25 July 2008 Diagnostic Pathology 2008, 3:31 doi:10.1186/1746-1596-3-31 This article is available from: http://www.diagnosticpathology.org/content/3/1/31 © 2008 Grayson and Pantanowitz; licensee BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Abstract This review provides a comprehensive overview of the broad clinicopathologic spectrum of cutaneous Kaposi sarcoma (KS) lesions. Variants discussed include: usual KS lesions associated with disease progression (i.e. patch, plaque and nodular stage); morphologic subtypes alluded to in the older literature such as anaplastic and telangiectatic KS, as well as several lymphedematous variants; and numerous recently described variants including hyperkeratotic, keloidal, micronodular, pyogenic granuloma-like, ecchymotic, and intravascular KS. Involuting lesions as a result of treatment related regression are also presented. Introduction progression, (2) KS variants alluded to in the older litera- Kaposi sarcoma (KS) is a vascular lesion of low-grade ture, (3) more recently described KS variants, and (4) KS malignant potential that presents most frequently with lesions as a consequence of therapy. -

Multinucleate Cell Angiohistiocytoma
To protect the rights of the author(s) and publisher we inform you that this PDF is an uncorrected proof for internal business use only by the author(s), editor(s), reviewer(s), Elsevier and typesetter Toppan Best-set. It is not allowed to publish this proof online or in print. This proof copy is the copyright property of the publisher and is confidential until formal publication. These proofs may contain color(colour) figures. Those figures may print black and white in the final printed book if a color(colour) print product has not been planned. The color(colour) figures will appear in color(colour) in all electronic versions of this book. s0060 MULTINUCLEATE CELL ANGIOHISTIOCYTOMA s0065 Definition • Fibroblast-like and histiocyte-like mononuclear cells u0390 p0300 • A distinctive benign dermal proliferation composed • Thickened collagen bundles, frequently hyalinized u0395 of thin-walled capillaries and veins, admixed with • Occasional inflammatory cells, predominantly u0400 scattered multinucleated cells lymphocytes • Hemorrhage absent, no hemosiderin deposition u0405 s0070 Clinical features • Decreased elastic fibers in the dermis can be observed u0410 s0075 Epidemiology • Overlying epidermis normal, but can also be u0415 p0310 • Female predominance (F:M = 3 : 1) hyperplastic u0275 • Middle-aged adult patients • Proliferation restricted to upper and middermis u0420 s0080 Presentation Immunopathology/special stains s0100 p0325 • Slowly growing single or multiple firm, red-brown to • Multinucleated cells display variable CD68 positivity -

Requires Subscription
DERMATOLOGY PRACTICAL & CONCEPTUAL www.derm101.com An unusual lesion on the nose: microvenular hemangioma A. Tulin Mansur1, Gulsen Tukenmez Demirci2, Eltaf A. Ozbal Koc3, Semsi Yildiz4 1 Dermatology Department, Istanbul Hospital, Baskent University, Istanbul, Turkey 2 Dermatology Department, Acibadem Altunizade Hospital, Acibadem University, Istanbul, Turkey 3 Ear, Nose and Throat Diseases Department, Istanbul Hospital, Baskent University, Istanbul, Turkey 4 Pathology Department, Istanbul Hospital, Baskent University, Istanbul, Turkey Key words: vascular anomaly, hemangioma Citation: Mansur AT, Tukenmez Demirci G, Ozbal Koc EA, Yildiz S. An unusual lesion on the nose: microvenular hemangioma. Dermatol Pract Concept. 2018;8(1):7-11. DOI: https://doi.org/10.5826/dpc.0801a02 Received: April 25, 2017; Accepted: October 6, 2017; Published: January 31, 2018 Copyright: ©2018 Mansur et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Funding: None. Competing interests: The authors have no conflicts of interest to disclose. All authors have contributed significantly to this publication. Corresponding author: Gulsen Tukenmez Demirci, Associate Professor, Dermatology Department, Acibadem Altunizade Hospital, Üsküdar 34662, Istanbul,˙ Turkey. Email: [email protected] ABSTRACT Microvenular hemangioma (MVH) is an acquired, benign type of hemangioma that usually manifests itself as a solitary, slowly growing, red to violaceous, asymptomatic papule, plaque or nodule. It is typically located on the trunk or extremities of young adults. It can be difficult to differentiate MVH from other types of hemangioma and Kaposi sarcoma. Herein we report a case of MVH unusual for its location, age of onset, and morphologic features. -

UC Davis Dermatology Online Journal
UC Davis Dermatology Online Journal Title Erythematous asymptomatic nodule on the sole Permalink https://escholarship.org/uc/item/9969k7kp Journal Dermatology Online Journal, 23(10) Authors Paret-Sanz, Cristina del Alcázar-Viladomiu, Elena Pérez-Muñoz, Noelia et al. Publication Date 2017 DOI 10.5070/D32310037003 License https://creativecommons.org/licenses/by-nc-nd/4.0/ 4.0 Peer reviewed eScholarship.org Powered by the California Digital Library University of California Volume 23 Number 10 | October 2017 Dermatology Online Journal || Case Presentation DOJ 23 (10): 12 Erythematous asymptomatic nodule on the sole Cristina Paret-Sanz1 MD, Elena del Alcázar-Viladomiu MD, Noelia Pérez-Muñoz2 MD, Ana Iglesias-Plaza1 MD, Montserrat Salleras-Redonnet1 MD PhD Affiliations: 1Department of Dermatology, Hospital Universitari Sagrat Cor, Barcelona, Spain, 2Department of Pathology, Hospital Universitari Sagrat Cor, Barcelona, Spain Corresponding Author: Cristina Paret Sanz MD, Department of Dermatology, Hospital Universitari Sagrat Cor, Carrer de Viladomat, 288, 08029 Barcelona, Spain, Email address: [email protected] Abstract A 75-year-old man presented to the dermatology clinic with an asymptomatic lesion on his right plantar surface. The lesion had progressively grown for two months. Physical examination revealed an erythematous and slightly scaly nodule measuring 10x10 mm. Dermoscopy examination showed central diffuse erythema with small red globules. A punch- biopsy revealed a proliferation of irregularly branched small vessels with collapsed lumen, extending in an infiltrative pattern in the superficial and deep dermis. Although this is a rare location, a diagnosis of microvenular hemangioma was made. Keywords: vascular neoplasm; microvenular hemangioma, hemangioma Introduction Microvenular hemangioma is a rare, benign, acquired vascular tumor, which usually occurs as a solitary slowly enlarging, red, purple, or reddish-blue plaque or nodule of less than 30 mm in diameter. -

Table of Contents
CONTENTS 1. Specimen evaluation 1 Specimen Type. 1 Clinical History. 1 Radiologic Correlation . 1 Special Studies . 1 Immunohistochemistry . 2 Electron Microscopy. 2 Genetics. 3 Recognizing Non-Soft Tissue Tumors. 3 Grading and Prognostication of Sarcomas. 3 Management of Specimen and Reporting. 4 2. Nonmalignant Fibroblastic and Myofibroblastic Tumors and Tumor-Like Lesions . 7 Nodular Fasciitis . 7 Proliferative Fasciitis and Myositis . 12 Ischemic Fasciitis . 18 Fibroma of Tendon Sheath. 21 Nuchal-Type Fibroma . 24 Gardner-Associated Fibroma. 27 Desmoplastic Fibroblastoma. 27 Elastofibroma. 34 Pleomorphic Fibroma of Skin. 39 Intranodal (Palisaded) Myofibroblastoma. 39 Other Fibroma Variants and Fibrous Proliferations . 44 Calcifying Fibrous (Pseudo)Tumor . 47 Juvenile Hyaline Fibromatosis. 52 Fibromatosis Colli. 54 Infantile Digital Fibroma/Fibromatosis. 58 Calcifying Aponeurotic Fibroma. 63 Fibrous Hamartoma of Infancy . 65 Myofibroma/Myofibromatosis . 69 Palmar/Plantar Fibromatosis. 80 Lipofibromatosis. 85 Diffuse Infantile Fibromatosis. 90 Desmoid-Type Fibromatosis . 92 Benign Fibrous Histiocytoma (Dermatofibroma). 98 xi Tumors of the Soft Tissues Non-neural Granular Cell Tumor. 104 Neurothekeoma . 104 Plexiform Fibrohistiocytic Tumor. 110 Superficial Acral Fibromyxoma . 113 Superficial Angiomyxoma (Cutaneous Myxoma). 118 Intramuscular Myxoma. 125 Juxta-articular Myxoma. 128 Aggressive Angiomyxoma . 128 Angiomyofibroblastoma. 135 Cellular Angiofibroma. 136 3. Fibroblastic/Myofibroblastic Neoplasms with Variable Biologic Potential. -
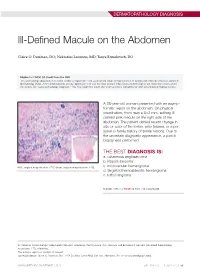
Ill-Defined Macule on the Abdomen
DERMATOPATHOLOGY DIAGNOSIS Ill-Defined Macule on the Abdomen Claire O. Dorfman, DO; Nektarios Lountzis, MD; Tanya Ermolovich, DO Eligible for 1 MOC SA Credit From the ABD This Dermatology Diagnosis in our print edition is eligible for 1 self-assessment credit for Maintenance of Certification from the American Board of Dermatology (ABD). After completing this activity, diplomates can visit the ABD website (http://www.abderm.org) to self-report the credits under the activity title “Cutis Dermatology Diagnosis.” You may report the credit after each activity is completed or after accumulating multiple credits. A 38-year-old woman presented with an asymp- tomatic lesion on the abdomen. On physical examination, there was a 5×2-mm, solitary, ill- defined pink macule on the right side of the abdomen. The patient denied recent change in size or color of the lesion, prior trauma, or a per- sonal or family history of similar lesions. Due to the uncertain diagnostic appearance, a punch biopsy was performed. THE BEST DIAGNOSIS IS: a. cutaneous angiosarcoma b. Kaposi sarcoma H&E, original magnification ×200 (inset, original magnification ×40). c. microvenular hemangioma d. targetoid hemosiderotic hemangioma e. tufted angioma PLEASE TURN TO PAGE 68 FOR THE DIAGNOSIS Dr. Dorfman is from Lehigh Valley Health Network, Allentown, Pennsylvania. Drs. Lountzis and Ermolovich are from Advanced Dermatology Associates, LTD, Allentown. The authors report no conflict of interest. Correspondence: Claire O. Dorfman, DO, 1259 S Cedar Crest Blvd, Ste 100, Allentown, PA 18103 ([email protected]). WWW.MDEDGE.COM/DERMATOLOGY VOL. 104 NO. 1 I JULY 2019 53 DERMATOPATHOLOGY DIAGNOSIS DISCUSSION THE DIAGNOSIS: Microvenular Hemangioma icrovenular hemangioma is an acquired benign vas- from pink patches to dark purple plaques or nodules that cular neoplasm that was described by Hunt et al1 in commonly occur on the lower legs10; however, the clinical M1991, though Bantel et al2 reported a similar entity appearance of KS varies depending on the clinical variant termed micropapillary angioma in 1989. -

Pathology of Vascular Skin Lesions
122 Sangüeza and Requena / Pathology of Vascular Skin Lesions 13. Albrecht S, Kahn HJ. Immunohistochemistry of intravascular papillary endothelial hyperplasia. J Cutan Pathol 1990;17:16–21. 14. Rosai J, Akerman LR. Intravascular atypical vascular proliferation. Arch Dermatol 1974;109:704–17. 15. Cooper PH. Vascular tumors. In: Farmer R, Hood AF, eds. Pathology of the Skin. Norwalk, CT, Appleton & Lange, 1990:804–46. 16. Pins MR, Rosenthal DI, Springfield DS, Rosemberg AE. Florid extravascular papillary endothelial hyperplasia (Masson’s pseudoangiosarcoma) presenting as a soft-tissue sarcoma. Arch Pathol Lab Med 1993;117:259–63. 17. Chen KT. Extravascular papillary endothelial hyperplasia. J Surg Oncol 1987;36:52–4. 18. Borrelli L, Ciniglio M, Faffulli N, Del Torto M. Intravascular papillary endothelial hyperplasia in the hand of a fencer. Pathologica 1992;84:551–6. 19. Inaloz HS, Patel G, Knight AG. Recurrent intravascular papillary endothelial hyperplasia developing from a pyogenic granuloma. J Eur Acad Dermatol Venereol 2000;15:156–8. 20. Hashimoto H, Daimaru Y, Enjoji M. Intravascular papillary endothelial hyperplasia. A clinicopatho- logic study of 91 cases. Am J Dermatopathol 1983;5:539–46. 21. Heyden G, Dahl I, Angervall L. Intravascular papillary endothelial hyperplasia in the oral mucosa. Oral Surg Oral Med Oral Pathol 1978;45:83–7. 22. Buchner A, Merrell PW, Carpenter WM, Leider AS. Oral intravascular papillary endothelial hyperpla- sia. J Oral Pathol Med 1990;19:419–22. 23. Stern Y, Braslavsky D, Shpitzer T, Feinmesser R. Papillary endothelial hyperplasia in the tongue: a benign lesion that may be mistaken for angiosarcoma. J Otolaryngol 1994;23:81–3. -

2014 Slide Library Case Summary Questions & Answers With
2014 Slide Library Case Summary Questions & Answers with Discussions 51st Annual Meeting November 6-9, 2014 Chicago Hilton & Towers Chicago, Illinois The American Society of Dermatopathology ARTHUR K. BALIN, MD, PhD, FASDP FCAP, FASCP, FACP, FAAD, FACMMSCO, FASDS, FAACS, FASLMS, FRSM, AGSF, FGSA, FACN, FAAA, FNACB, FFRBM, FMMS, FPCP ASDP REFERENCE SLIDE LIBRARY November 2014 Dear Fellows of the American Society of Dermatopathology, The American Society of Dermatopathology would like to invite you to submit slides to the Reference Slide Library. At this time there are over 9300 slides in the library. The collection grew 2% over the past year. This collection continues to grow from member’s generous contributions over the years. The slides are appreciated and are here for you to view at the Sally Balin Medical Center. Below are the directions for submission. Submission requirements for the American Society of Dermatopathology Reference Slide Library: 1. One H & E slide for each case (if available) 2. Site of biopsy 3. Pathologic diagnosis Not required, but additional information to include: 1. Microscopic description of the slide illustrating the salient diagnostic points 2. Clinical history and pertinent laboratory data, if known 3. Specific stain, if helpful 4. Clinical photograph 5. Additional note, reference or comment of teaching value Teaching sets or collections of conditions are especially useful. In addition, infrequently seen conditions are continually desired. Even a single case is helpful. Usually, the written submission requirement can be fulfilled by enclosing a copy of the pathology report prepared for diagnosis of the submitted case. As a guideline, please contribute conditions seen with a frequency of less than 1 in 100 specimens. -

Contents SECTION 1 Fibrohistiocytic Tumors 1 Classification Of
Contents SECTION 1 Fibrohistiocytic Tumors 1 Classification of Fibrohistiocytic Tumors 2 Dermatofibroma 3 Juvenile Xanthogranuloma 4 Solitary Reticulohistiocytoma and Multicentric Reticulohistiocytosis 5 Acrochordons and Pendulous Fibromas 6 Cutaneous Solitary Fibrous Tumor 7 Sclerotic Fibroma (Storiform Collagenoma) 8 Plaque-Like CD34-Positive Dermal Fibroma (Medallion-Like Dermal Dendrocyte Hamartoma) 9 Desmoplastic Fibroblastoma (Collagenous Fibroma) 10 Pleomorphic Fibroma of the Skin 11 Fibroma of Tendon Sheath 12 Giant Cell Tumor of Tendon Sheath 13 Cutaneous Myxoma 14 Superficial Acral Fibromyxoma 15 Cellular Digital Fibroma 16 Nuchal Fibroma and Gardner Syndrome–Associated Fibroma 17 Cerebriform Collagenoma Associated with Proteus Syndrome 18 Elastofibroma 19 Nodular Fasciitis, Proliferative Fasciitis, and Ischemic Fasciitis 20 Fibromatosis 21 Juvenile Hyaline Fibromatosis 22 Fibrous Hamartoma of Infancy 23 Calcifying Aponeurotic Fibroma 24 Dermatofibrosarcoma Protuberans 25 Angiomatoid Fibrous Histiocytoma 26 Plexiform Fibrohistiocytic Tumor 27 Soft Tissue Giant Cell Tumor of Low Malignant Potential 28 Inflammatory Myofibroblastic Tumor 29 Inflammatory Myxohyaline Tumor of Distal Extremities 30 Atypical Fibroxanthoma 31 Malignant Fibrous Histiocytoma 32 Fibrosarcoma 33 Epithelioid Sarcoma 34 Synovial Sarcoma 35 Alveolar Soft Part Sarcoma SECTION 2 Myofibroblastic and Muscle Tumors 36 Myofibroblasts, Muscle Cells, and Classification of Skin Proliferations with Myofibroblastic and Muscle Differentiation 37 Infantile Myofibromatosis -

Multiple Microvenular Hemangioma: a Case Report
ONCOLOGY LETTERS 7: 275-277, 2014 Multiple microvenular hemangioma: A case report DONG FANG AI1, YAN LI2, AIKAJ JINDAL3 and PING LI1 1Department of Dermatology, Cangzhou Central Hospital, Cangzhou, Hebei 061001; 2Department of Dermatology, Tianjin Medical University, General Hospital; 3Tianjin Medical University, Tianjin 300052, P.R. China Received April 20, 2013; Accepted October 25, 2013 DOI: 10.3892/ol.2013.1659 Abstract. The current study reports a case of multiple of their presence on the trunk and limbs for the past 5 years microvenular hemangioma (MH). A 35-year-old male and with the absence of any precipitating factor. Initially, presented with dark red maculopapules on the trunk and limbs the lesions were the size of rice granules, painless and itchy, that had been apparent for 5 years. The number of lesions although not troublesome enough to persuade the patient to exceeded 100 in total. A histological examination demon- attend a consultation. Thereafter, the size of the lesions gradu- strated multiple, irregular, branching venules in the dermis, ally enlarged and also increased in number. Subsequently, the without any endothelial atypia. On immunohistochemical individual sought medical advice at a local hospital and was analysis of the vascular structures, the endothelial cells stained prescribed oral antihistamines and topical corticosteroids that positive for CD31, CD34 and factor VIII, and the perivascular proved of no benefit. No other family member demonstrated cells stained positive for SMA and HHF-35. These observa- such lesions. tions were consistent with a diagnosis of MH, and should be differentiated from the most common differential diagnosis Lesion examination. Upon examination, numerous dark of patch-stage Kaposi's sarcoma. -

ISSVA Classification
Cambridge University Press 978-0-521-84851-0 - Color Atlas of Vascular Tumors and Vascular Malformations Odile Enjolras, Michel Wassef and Rene Chapot Excerpt More information Introduction: ISSVA Classification © Cambridge University Press www.cambridge.org Cambridge University Press 978-0-521-84851-0 - Color Atlas of Vascular Tumors and Vascular Malformations Odile Enjolras, Michel Wassef and Rene Chapot Excerpt More information The International Society for the Study of Vascular Anomalies (ISSVA) was born in 1992 after 16 years of biennial international workshops. Interdisciplinary and international collaboration has been the guiding principle of the ISSVA, with a primary goal of improving our understanding and management of these lesions. This continuing workshop has taken place every two years in various countries around the world. Multiple nomenclatures for ‘‘angiomas’’ or ‘‘vascular birthmarks’’ have long been an important obstacle to communication amongst the various medical specialists (pediatricians, dermatologists, surgeons, radiologists, angiologists, ophthalmologists, ENT surgeons, pathologists, etc.) involved in the management of these patients (13). During discussions among members of the workshop it was decided to discard the old terms ‘‘angioma’’ and ‘‘birthmark.’’ A very basic classification system was adopted by the ISSVA during its 1996 workshop, to give us a common language. We now distinguish two main types of vascular anomalies: vascular tumors (the most common type is infantile hemangioma, but other rare vascular tumors occur in children as well as in adults) and vascular malformations (10). This system is based on the founding biological investigation of Mulliken and Glowacki published in 1982, which provided the groundwork for a proper identification of vascular birthmarks (16). -

Vascular Malformations and Tumors Continues to Grow Overview Table Vascular Anomalies
ISSVA classification for vascular anomalies © (Approved at the 20th ISSVA Workshop, Melbourne, April 2014, last revision May 2018) This classification is intended to evolve as our understanding of the biology and genetics of vascular malformations and tumors continues to grow Overview table Vascular anomalies Vascular tumors Vascular malformations of major named associated with Simple Combined ° vessels other anomalies Benign Capillary malformations CVM, CLM See details See list Lymphatic malformations LVM, CLVM Locally aggressive or borderline Venous malformations CAVM* Arteriovenous malformations* CLAVM* Malignant Arteriovenous fistula* others °defined as two or more vascular malformations found in one lesion * high-flow lesions A list of causal genes and related vascular anomalies is available in Appendix 2 The tumor or malformation nature or precise classification of some lesions is still unclear. These lesions appear in a separate provisional list. For more details, click1 on Abbreviations used the underlined links Back to ISSVA classification of vascular tumors 1a Type Alt overview for previous view Benign vascular tumors 1 Infantile hemangioma / Hemangioma of infancy see details Congenital hemangioma GNAQ / GNA11 Rapidly involuting (RICH) * Non-involuting (NICH) Partially involuting (PICH) Tufted angioma * ° GNA14 Spindle-cell hemangioma IDH1 / IDH2 Epithelioid hemangioma FOS Pyogenic granuloma (also known as lobular capillary hemangioma) BRAF / RAS / GNA14 Others see details * some lesions may be associated with thrombocytopenia