H. Sleumer Leyden) Spiral Pseudo-Whorls, Subopposite
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Macadamia Integrifolia HAES 741)
bioRxiv preprint doi: https://doi.org/10.1101/2020.05.25.114009; this version posted May 27, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. Chromosome-scale assembly and annotation of the macadamia genome (Macadamia integrifolia HAES 741) Catherine J. Nock1†*, Abdul Baten1,2†, Ramil Mauleon1, Kirsty S. Langdon1, Bruce Topp3, Craig Hardner3, Agnelo Furtado3, Robert J. Henry3 and Graham J. King1 1Southern Cross Plant Science, Southern Cross University, Lismore NSW 2480, Australia 2AgResearch NZ, Grasslands Research Centre, Palmerston North 4442, New Zealand 3Queensland Alliance for Agriculture and Food Innovation, University of Queensland, St Lucia QLD 4069, Australia †These authors contributed equally to this work *Corresponding author, [email protected] ORCID 0000-0001-5609-4681 Abstract Macadamia integrifolia is a representative of the large basal eudicot family Proteaceae and the main progenitor species of the Australian native nut crop macadamia. Since its commercialisation in Hawaii fewer than 100 years ago, global production has expanded rapidly. However, genomic resources are limited in comparison to other horticultural crops. The first draft assembly of M. integrifolia had good coverage of the functional gene space but its high fragmentation has restricted its use in comparative genomics and association studies. Here we have generated an improved assembly of cultivar HAES 741 (4,094 scaffolds, 745 Mb, N50 413 kb) using a combination of Illumina paired and PacBio long read sequences. -

Method to Estimate Dry-Kiln Schedules and Species Groupings: Tropical and Temperate Hardwoods
United States Department of Agriculture Method to Estimate Forest Service Forest Dry-Kiln Schedules Products Laboratory Research and Species Groupings Paper FPL–RP–548 Tropical and Temperate Hardwoods William T. Simpson Abstract Contents Dry-kiln schedules have been developed for many wood Page species. However, one problem is that many, especially tropical species, have no recommended schedule. Another Introduction................................................................1 problem in drying tropical species is the lack of a way to Estimation of Kiln Schedules.........................................1 group them when it is impractical to fill a kiln with a single Background .............................................................1 species. This report investigates the possibility of estimating kiln schedules and grouping species for drying using basic Related Research...................................................1 specific gravity as the primary variable for prediction and grouping. In this study, kiln schedules were estimated by Current Kiln Schedules ..........................................1 establishing least squares relationships between schedule Method of Schedule Estimation...................................2 parameters and basic specific gravity. These relationships were then applied to estimate schedules for 3,237 species Estimation of Initial Conditions ..............................2 from Africa, Asia and Oceana, and Latin America. Nine drying groups were established, based on intervals of specific Estimation -

NSW Rainforest Trees Part
This document has been scanned from hard-copy archives for research and study purposes. Please note not all information may be current. We have tried, in preparing this copy, to make the content accessible to the widest possible audience but in some cases we recognise that the automatic text recognition maybe inadequate and we apologise in advance for any inconvenience this may cause. · RESEARCH NOTE No. 35 ~.I~=1 FORESTRY COMMISSION OF N.S.W. RESEARCH NOTE No. 35 P)JBLISHED 197R N.S.W. RAINFOREST TREES PART VII FAMILIES: PROTEACEAE SANTALACEAE NYCTAGINACEAE GYROSTEMONACEAE ANNONACEAE EUPOMATIACEAE MONIMIACEAE AUTHOR A.G.FLOYD (Research Note No. 35) National Library of Australia card number and ISBN ISBN 0 7240 13997 ISSN 0085-3984 INTRODUCTION This is the seventh in a series ofresearch notes describing the rainforest trees of N.S. W. Previous publications are:- Research Note No. 3 (I 960)-N.S.W. Rainforest Trees. Part I Family LAURACEAE. A. G. Floyd and H. C. Hayes. Research Note No. 7 (1961)-N.S.W. Rainforest Trees. Part II Families Capparidaceae, Escalloniaceae, Pittosporaceae, Cunoniaceae, Davidsoniaceae. A. G. Floyd and H. C. Hayes. Research Note No. 28 (I 973)-N.S.W. Rainforest Trees. Part III Family Myrtaceae. A. G. Floyd. Research Note No. 29 (I 976)-N.S.W. Rainforest Trees. Part IV Family Rutaceae. A. G. Floyd. Research Note No. 32 (I977)-N.S.W. Rainforest Trees. Part V Families Sapindaceae, Akaniaceae. A. G. Floyd. Research Note No. 34 (1977)-N.S.W. Rainforest Trees. Part VI Families Podocarpaceae, Araucariaceae, Cupressaceae, Fagaceae, Ulmaceae, Moraceae, Urticaceae. -

Brooklyn, Cloudland, Melsonby (Gaarraay)
BUSH BLITZ SPECIES DISCOVERY PROGRAM Brooklyn, Cloudland, Melsonby (Gaarraay) Nature Refuges Eubenangee Swamp, Hann Tableland, Melsonby (Gaarraay) National Parks Upper Bridge Creek Queensland 29 April–27 May · 26–27 July 2010 Australian Biological Resources Study What is Contents Bush Blitz? Bush Blitz is a four-year, What is Bush Blitz? 2 multi-million dollar Abbreviations 2 partnership between the Summary 3 Australian Government, Introduction 4 BHP Billiton and Earthwatch Reserves Overview 6 Australia to document plants Methods 11 and animals in selected properties across Australia’s Results 14 National Reserve System. Discussion 17 Appendix A: Species Lists 31 Fauna 32 This innovative partnership Vertebrates 32 harnesses the expertise of many Invertebrates 50 of Australia’s top scientists from Flora 62 museums, herbaria, universities, Appendix B: Threatened Species 107 and other institutions and Fauna 108 organisations across the country. Flora 111 Appendix C: Exotic and Pest Species 113 Fauna 114 Flora 115 Glossary 119 Abbreviations ANHAT Australian Natural Heritage Assessment Tool EPBC Act Environment Protection and Biodiversity Conservation Act 1999 (Commonwealth) NCA Nature Conservation Act 1992 (Queensland) NRS National Reserve System 2 Bush Blitz survey report Summary A Bush Blitz survey was conducted in the Cape Exotic vertebrate pests were not a focus York Peninsula, Einasleigh Uplands and Wet of this Bush Blitz, however the Cane Toad Tropics bioregions of Queensland during April, (Rhinella marina) was recorded in both Cloudland May and July 2010. Results include 1,186 species Nature Refuge and Hann Tableland National added to those known across the reserves. Of Park. Only one exotic invertebrate species was these, 36 are putative species new to science, recorded, the Spiked Awlsnail (Allopeas clavulinus) including 24 species of true bug, 9 species of in Cloudland Nature Refuge. -

IV. on the Proteaceć of Jussieu. by Mr. Robert Brown, Lib. LS
IV. On the Proteacea of Jussieu. By -Mr. Robert Brown, Lib. L.S. Read Jan. 17, 1809. THELinnean system of botany, though confessedly artificial, has not only contributed more than all others to facilitate tlie knowledge of species, but, by constantly directing the attention to those essential parts of the flower on which it is founded, has made us acquainted with more of their important modific-a t’ ions than we probably should have known, had it not been generally adopted, and has thus laid a more solid foundation for the esta- blishment of a natural arrangement, the superior importance of which no one has been inore fully impressed with than Linnzus hiinself. There are still, however, certain circumstances respccting the stamina and pistilla, which appear to iiie to havc been much less attended to than they deserve, both by Linneus and succeeding botanists. What I chiefly allude to is the state of these organs before the expansion of the flower. Tlie utility of ascertaining the internal condition of the ovarium before fecundation will liardly be called in question, now that the immortal worlis of Gxrtner and Jussieu hare demonstrated the necessity of minutely studying the fruits of plants in attempting to arrange tlicin ac- cording to tlic sum of their affinities, as in many cases the true nature of tlie ripc fruit, cspecially witli respect to the placenta- tion of the seeds, can oiily be detcrniined by this mc;~ns. Its importance is indeed expressly inculcated by many l~ot:inists, Tf’llO, 16 Mr. BROWN,on the Proteacee of Jussieu. -
Abelmoschus Moschatus Subsp
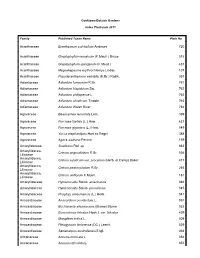
Cooktown Botanic Gardens Index Plantarum 2011 Family Published Taxon Name Plate No Acanthaceae Eranthemum pulchellum Andrews 720 Acanthaceae Graptophyllum excelsum (F.Meull.) Druce 515 Acanthaceae Graptophyllum spinigerum (F.Meull.) 437 Acanthaceae Megaskepasma erythrochlamys Lindau 107 Acanthaceae Pseuderanthemum variabile (R.Br.) Radlk. 357 Adiantaceae Adiantum formosum R.Br. 761 Adiantaceae Adiantum hispidulum Sw. 762 Adiantaceae Adiantum philippense L. 765 Adiantaceae Adiantum silvaticum Tindale 763 Adiantaceae Adiantum Walsh River 764 Agavaceae Beaucarnea recurvata Lem. 399 Agavaceae Furcraea foetida (L.) Haw. 637 Agavaceae Furcraea gigantea (L.) Haw. 049 Agavaceae Yucca elephantipes Hort.ex Regel 388 Agavaceae Agave sisalana Perrine. 159 Amarylidaceae Scadoxus Raf. sp 663 Amaryllidacea, Crinum angustifolium R.Br. 536 Liliaceae Amaryllidacea, Crinum asiaticum var. procerum (Herb. et Carey) Baker 417 Liliaceae Amaryllidacea, Crinum pedunculatum R.Br. 265 Liliaceae Amaryllidacea, Crinum uniflorum F.Muell. 161 Liliaceae Amaryllidaceae Hymenocallis Salisb. americanus 046 Amaryllidaceae Hymenocallis Salisb. peruvianna 045 Amaryllidaceae Proiphys amboinensis (L.) Herb. 041 Anacardiaceae Anacardium occidentale L. 051 Anacardiaceae Buchanania arborescens (Blume) Blume. 022 Anacardiaceae Euroschinus falcatus Hook.f. var. falcatus 429 Anacardiaceae Mangifera indica L. 009 Anacardiaceae Pleiogynium timorense (DC.) Leenh. 029 Anacardiaceae Semecarpus australiensis Engl. 368 Annonaceae Annona muricata L. 054 Annonaceae Annona reticulata L. 053 Annonaceae Annona squamosa 602 Annonaceae Cananga odorata (Lam.) Hook.f.&Thomson 406 Annonaceae Melodorum leichhardtii (F.Muell.) Diels. 360 Annonaceae Rollinia deliciosa Saff. 098 Apiaceae Centella asiatica (L.) Urb. 570 Apocynaceae Adenium obesum (Forssk.) Roem. & Schult. 489 Apocynaceae Allamanda cathartica L. 047 Apocynaceae Allamanda violacea Gardn. & Field. 048 Apocynaceae Alstonia actinophylla (A.Cunn.) K.Schum. 026 Apocynaceae Alstonia scholaris (L.) R.Br. 012 Apocynaceae Alyxia ruscifolia R.Br. -

Edition 2 from Forest to Fjaeldmark the Vegetation Communities Highland Treeless Vegetation
Edition 2 From Forest to Fjaeldmark The Vegetation Communities Highland treeless vegetation Richea scoparia Edition 2 From Forest to Fjaeldmark 1 Highland treeless vegetation Community (Code) Page Alpine coniferous heathland (HCH) 4 Cushion moorland (HCM) 6 Eastern alpine heathland (HHE) 8 Eastern alpine sedgeland (HSE) 10 Eastern alpine vegetation (undifferentiated) (HUE) 12 Western alpine heathland (HHW) 13 Western alpine sedgeland/herbland (HSW) 15 General description Rainforest and related scrub, Dry eucalypt forest and woodland, Scrub, heathland and coastal complexes. Highland treeless vegetation communities occur Likewise, some non-forest communities with wide within the alpine zone where the growth of trees is environmental amplitudes, such as wetlands, may be impeded by climatic factors. The altitude above found in alpine areas. which trees cannot survive varies between approximately 700 m in the south-west to over The boundaries between alpine vegetation communities are usually well defined, but 1 400 m in the north-east highlands; its exact location depends on a number of factors. In many communities may occur in a tight mosaic. In these parts of Tasmania the boundary is not well defined. situations, mapping community boundaries at Sometimes tree lines are inverted due to exposure 1:25 000 may not be feasible. This is particularly the or frost hollows. problem in the eastern highlands; the class Eastern alpine vegetation (undifferentiated) (HUE) is used in There are seven specific highland heathland, those areas where remote sensing does not provide sedgeland and moorland mapping communities, sufficient resolution. including one undifferentiated class. Other highland treeless vegetation such as grasslands, herbfields, A minor revision in 2017 added information on the grassy sedgelands and wetlands are described in occurrence of peatland pool complexes, and other sections. -

Finschia-"A Genus of "Nut" Trees of the Southwest Pacific
Finschia-" A Genus of "Nut" Trees of the Southwest Pacific c. T. WHITE1 INTRODUCTION A PLANT FAMILY with a most interesting and F. Muell., Carnarvonia F. Muell., D arlin"gia F; intriguing distribution is Proteaceae, which finds Muell., Hollandaea F. Muell. (two spp.) , Mus its greatest development in Australia (650 " gravea F. Muell., and Placospermum White & species) on the one hand and South Africa (300 Francis. A surprising feature is the absence, species) on the other, though the two countries with the exception of one species in New Zea have no genera in common. Practically all the land, of the family "from Polynesia. South African species and the vast majority of There is in the islands of the southwest Paci "Australian ones are markedly xerophytic. The fic-Caroline Islands, New Guinea, Solomon largest genus, Greoillea R. Br., consists mainly Islands, and the New Hebrides-a group of trees of xerophytic shrubs or small trees but a few with the floral characters of Greuillea R. Br. are large trees found in the rain forests of and the fruit of Helicia Lour. These, I consider, tropical and subtropical eastern Australia, New all belong 'to Finschia Warb. This genus was Guinea, and New Caledonia. In the southwest founded by Warburg (1891: 297 ) on"a tree Pacific area the family finds its greatest develop from northeastern New Guinea. His original ment in northeastern Australia, where trees be description would cover Grevillea R. Br. exactly longing to it provide the great bulk of cabinet though he does not mention this genus and on timbers known in the trade as "Silky Oaks." the following page the distinctions he gives for There is close affinity between the Proteaceae of separating his proposed new genus from H elicia eastern Australia and of western South America are exactly those which distinguish Greuillea as illustrated by the genera Embothrium Forst. -

Newsletter No.100
AssociationAustralian of NativeSocieties Plants for Growing Society (Australia)Australian IncPlants Ref No. ISSN 0725-8755 Newsletter No. 100 – February 2015 GSG Vic Programme 2015 GSG SE Qld Programme 2015 Leader: Neil Marriott Meetings are usually held on the last Sunday 693 Panrock Reservoir Rd, Stawell, Vic. 3380 of the even months. We meet for a communal p 03 5356 2404 or 0458 177 989 morning tea at 9.30am after which the meetings e [email protected] commence at 10.00am. Visitors are always welcome. For more information or to check venues Contact Neil for queries about program for the etc please contact Bryson Easton on 0402 242 180 year. Any members who would like to visit the or Noreen Baxter on (07) 3871 3932 as changes official collection, obtain cutting material or seed, can occur. assist in its maintenance, and stay in our cottage for a few days are invited to contact Neil. Sunday, 22 February Venue: Home of Gail and Adrian Wockner, 5 Horizon Drive, Highfields Qld 4352 Time: 9:30am for 10am meeting Newsletter No. 100 No. Newsletter GSG NSW Programme 2015 Monday, 27 April For details contact Peter Olde 02 4659 6598. Venue: Mt Coot-tha Botanic Garden – meeting in the picnic sheds where road becomes two way 9:30am for 10am meeting Special thanks to the Victorian and New South Wales Time: chapters for this edition of the newsletter. Queensland Topic: Review of the Grevillea Gardens members, please note deadlines on back page for Note the change to Monday is so that members the following newsletter. -

Approved NSW & National Recovery Plan Eidothea Hardeniana
Approved NSW & National Recovery Plan Eidothea hardeniana September 2004 © Department of Environment and Conservation (NSW), July 2004. This work is copyright. However, material presented in this plan may be copied for personal use or published for educational purposes, providing that any extracts are fully acknowledged. Apart from this and any other use as permitted under the Copyright Act 1968, no part may be reproduced without prior written permission from NSW Department of Environment and Conservation. NSW Department of Environment and Conservation 43 Bridge Street (PO Box 1967) Hurstville NSW 2220 Tel: 02 9585 6444 www.nationalparks.nsw.gov.au Requests for information or comments regarding the recovery program for the Nightcap Oak are best directed to: The Nightcap Oak Recovery Co-ordinator Threatened Species Unit, North East Branch NSW Department of Environment and Conservation Locked Bag 914 Coffs Harbour NSW 2450 Tel: 02 6651 5946 Cover illustrator: Lesley Elkan © Botanic Gardens Trust, Sydney Cover illustration: Adult and juvenile leaves and fruit of Eidothea hardeniana This plan should be cited as follows: NSW Department of Environment and Conservation 2004, Recovery Plan for the Nightcap Oak (Eidothea hardeniana), Department of Environment and Conservation (NSW), Hurstville. ISBN 0 7313 6781 2 Recovery Plan The Nightcap Oak Recovery Plan for the Nightcap Oak (Eidothea hardeniana) Foreword The New South Wales Government established a new environment agency on 24 September 2003, the Department of Environment and Conservation (NSW), which incorporates the New South Wales National Parks and Wildlife Service. Responsibility for the preparation of Recovery Plans now rests with this new department. This document constitutes the New South Wales State and National Recovery Plan for Eidothea hardeniana Weston & Kooyman (Nightcap Oak), and as such considers the conservation requirements of the species across its range. -

Grow Natives in Pots
ATG About the Garden ATGFACTAbout SHEET the Garden Fact Sheet No. 21 GROWING NATIVES IN POTS The key factors to successfully growing Australian plants in containers are exactly the same as for exotic plants - drainage and nutrition. The main points to remember are that good drainage is even more important for certain natives (mainly those originating in arid areas with very light, sandy soils) and that plants in the Proteaceae family require low phosphorus fertilisers. When growing plants in containers it is important *PLANTS IN THE to know their type and origin so ask yourself the following questions: FAMILY PROTEACEAE • Tree, shrub or climber/groundcover? • Does it originate in a high or low rainfall area? INCLUDE: • Is its natural habitat sun or shade? • Natural soil type - light (sandy) or heavy (clay)? Athertonia banksia The type of plant determines the ultimate pot size. buckinghamia Knowing its origins will tell you the type of growing cardwellia medium you need, how much water and whether it darlingia should be placed in sun or shade. If the label doesn’t dryandra give you enough information, ask nursery staff. hakea lomatia Any type of container can be used, providing it has macadamia plenty of adequate sized drainage holes. Too small and oreocallis they will drain too slowly and block up easily; too large persoonia and all your precious water will run away too fast. Most petrophile, good quality terracotta and plastic pots come with stenocarpus and good drainage holes; a medium-sized pot should have telopea no less than four, about the size of a five cent piece. -

Evolutionary History of Floral Key Innovations in Angiosperms Elisabeth Reyes
Evolutionary history of floral key innovations in angiosperms Elisabeth Reyes To cite this version: Elisabeth Reyes. Evolutionary history of floral key innovations in angiosperms. Botanics. Université Paris Saclay (COmUE), 2016. English. NNT : 2016SACLS489. tel-01443353 HAL Id: tel-01443353 https://tel.archives-ouvertes.fr/tel-01443353 Submitted on 23 Jan 2017 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. NNT : 2016SACLS489 THESE DE DOCTORAT DE L’UNIVERSITE PARIS-SACLAY, préparée à l’Université Paris-Sud ÉCOLE DOCTORALE N° 567 Sciences du Végétal : du Gène à l’Ecosystème Spécialité de Doctorat : Biologie Par Mme Elisabeth Reyes Evolutionary history of floral key innovations in angiosperms Thèse présentée et soutenue à Orsay, le 13 décembre 2016 : Composition du Jury : M. Ronse de Craene, Louis Directeur de recherche aux Jardins Rapporteur Botaniques Royaux d’Édimbourg M. Forest, Félix Directeur de recherche aux Jardins Rapporteur Botaniques Royaux de Kew Mme. Damerval, Catherine Directrice de recherche au Moulon Président du jury M. Lowry, Porter Curateur en chef aux Jardins Examinateur Botaniques du Missouri M. Haevermans, Thomas Maître de conférences au MNHN Examinateur Mme. Nadot, Sophie Professeur à l’Université Paris-Sud Directeur de thèse M.