Practical Guidelines for Wetland Prairie Restoration in the Willamette Valley, Oregon
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Developing Biogeographically Based Population Introduction Protocols for At-Risk Willamette Valley Plant Species
Developing biogeographically based population introduction protocols for at-risk Willamette Valley plant species: Agrostis howellii (Howell’s bentgrass) Aster curtus (white-topped aster), Aster vialis (wayside aster), Delphinium leucophaeum (hot rock larkspur), Delphinium pavonaceaum (peacock larkspur), Erigeron decumbens var. decumbens (Willamette daisy), Horkelia congesta ssp. congesta (shaggy horkelia), Lomatium bradshawii (Bradshaw’s desert parsley), Lupinus sulphureus ssp. kincaidii (Kincaid’s lupine), Montia howellii (Howell’s montia), Sidalcea spp. (Willamette Valley checkermallows) Prepared by Steven D. Gisler Native Plant Conservation Program Oregon Department of Agriculture with contributions by Oregon Department of Agriculture staff for U.S. Fish and Wildlife Service Grant OR-EP-2, segment 13 Acknowledgements: We would like to thank the many people who contributed to the completion of this report. Thanks to Andy Robinson and Kathy Pendergrass (USFWS) for providing funding and encouragement (Grant no. OR-EP-2, segment 13). Kelly Amsberry, Rebecca Currin, and R.J. Meinke contributed to text completion and review, and Melissa Carr provided invaluable assistance in compiling data. Thanks also to the staff, interns and students who provided plant and habitat photos, and to Erin Amsberry Abood for assistance in final report preparation. Contact Information: Robert J. Meinke Kelly Amsberry Native Plant Conservation Program Native Plant Conservation Program Oregon Department of Agriculture Oregon Department of Agriculture Dept. of Botany and Plant Pathology Dept. of Botany and Plant Pathology Oregon State University Oregon State University Corvallis, OR 97331 Corvallis, OR 97331 (541) 737-2317 (541) 737-4333 [email protected] [email protected] Report format: The following species are presented in alphabetical order: Agrostis howellii (Howell’s bentgrass), Aster curtus (white-topped aster), Aster vialis (wayside aster), Delphinium leucophaeum (hot rock larkspur), Delphinium pavonaceaum (peacock larkspur), Erigeron decumbens var. -

Evolution of Angiosperm Pollen. 7. Nitrogen-Fixing Clade1
Evolution of Angiosperm Pollen. 7. Nitrogen-Fixing Clade1 Authors: Jiang, Wei, He, Hua-Jie, Lu, Lu, Burgess, Kevin S., Wang, Hong, et. al. Source: Annals of the Missouri Botanical Garden, 104(2) : 171-229 Published By: Missouri Botanical Garden Press URL: https://doi.org/10.3417/2019337 BioOne Complete (complete.BioOne.org) is a full-text database of 200 subscribed and open-access titles in the biological, ecological, and environmental sciences published by nonprofit societies, associations, museums, institutions, and presses. Your use of this PDF, the BioOne Complete website, and all posted and associated content indicates your acceptance of BioOne’s Terms of Use, available at www.bioone.org/terms-of-use. Usage of BioOne Complete content is strictly limited to personal, educational, and non - commercial use. Commercial inquiries or rights and permissions requests should be directed to the individual publisher as copyright holder. BioOne sees sustainable scholarly publishing as an inherently collaborative enterprise connecting authors, nonprofit publishers, academic institutions, research libraries, and research funders in the common goal of maximizing access to critical research. Downloaded From: https://bioone.org/journals/Annals-of-the-Missouri-Botanical-Garden on 01 Apr 2020 Terms of Use: https://bioone.org/terms-of-use Access provided by Kunming Institute of Botany, CAS Volume 104 Annals Number 2 of the R 2019 Missouri Botanical Garden EVOLUTION OF ANGIOSPERM Wei Jiang,2,3,7 Hua-Jie He,4,7 Lu Lu,2,5 POLLEN. 7. NITROGEN-FIXING Kevin S. Burgess,6 Hong Wang,2* and 2,4 CLADE1 De-Zhu Li * ABSTRACT Nitrogen-fixing symbiosis in root nodules is known in only 10 families, which are distributed among a clade of four orders and delimited as the nitrogen-fixing clade. -

Washington Plant List Douglas County by Scientific Name
The NatureMapping Program Washington Plant List Revised: 9/15/2011 Douglas County by Scientific Name (1) Non- native, (2) ID Scientific Name Common Name Plant Family Invasive √ 763 Acer glabrum Douglas maple Aceraceae 800 Alisma graminium Narrowleaf waterplantain Alismataceae 19 Alisma plantago-aquatica American waterplantain Alismataceae 1087 Rhus glabra Sumac Anacardiaceae 650 Rhus radicans Poison ivy Anacardiaceae 29 Angelica arguta Sharp-tooth angelica Apiaceae 809 Angelica canbyi Canby's angelica Apiaceae 915 Cymopteris terebinthinus Turpentine spring-parsley Apiaceae 167 Heracleum lanatum Cow parsnip Apiaceae 991 Ligusticum grayi Gray's lovage Apiaceae 709 Lomatium ambiguum Swale desert-parsley Apiaceae 997 Lomatium canbyi Canby's desert-parsley Apiaceae 573 Lomatium dissectum Fern-leaf biscuit-root Apiaceae 582 Lomatium geyeri Geyer's desert-parsley Apiaceae 586 Lomatium gormanii Gorman's desert-parsley Apiaceae 998 Lomatium grayi Gray's desert-parsley Apiaceae 999 Lomatium hambleniae Hamblen's desert-parsley Apiaceae 609 Lomatium macrocarpum Large-fruited lomatium Apiaceae 1000 Lomatium nudicaule Pestle parsnip Apiaceae 634 Lomatium triternatum Nine-leaf lomatium Apiaceae 474 Osmorhiza chilensis Sweet-cicely Apiaceae 264 Osmorhiza occidentalis Western sweet-cicely Apiaceae 1044 Osmorhiza purpurea Purple sweet-cicely Apiaceae 492 Sanicula graveolens Northern Sierra) sanicle Apiaceae 699 Apocynum androsaemifolium Spreading dogbane Apocynaceae 813 Apocynum cannabinum Hemp dogbane Apocynaceae 681 Asclepias speciosa Showy milkweed Asclepiadaceae -

DCDB: an Updated On-Line Database of Chromosome Numbers of Tribe Delphinieae (Ranunculaceae)
DCDB: an updated on-line database of chromosome numbers of tribe Delphinieae (Ranunculaceae) Maria Bosch1, Joan Simon1, Jordi López-Pujol2 & Cèsar Blanché1 1BioC-GReB, Laboratori de Botànica, Facultat de Farmàcia, Universitat de Barcelona. Av. Joan XXIII s/n. 08028 Barcelona, Catalonia (Spain) 2BioC-GReB, Botanic Institute of Barcelona (IBB-CSIC-ICUB). Passeig del Migdia s/n. 08028 Barcelona, Catalonia (Spain) VERSION 2.0 UPDATED 23/IV/2016 Abstract. A new version of the earlier chromosome database of tribe Delphinieae (Simon, J., M. Bosch, J. Molero & C. Blanché. 1999. A conspect of chromosome numbers in tribe Delphinieae (Ranunculaceae). Biodiversity Electronic Publications, 1 [Available online at http://hdl.handle.net/2445/95875]) is presented, after an accurate extensive literature and Internet survey, by adding the chromosome counts for the genera Aconitum L. (including Gymnaconitum (Stapf) Wei Wang & Z. D. Chen), Delphinium L. (including Staphisagria Spach), Consolida (DC.) S.F. Gray and Aconitella Spach, accumulated in the last 17 years. A total number of 2598 reports are presented, belonging to 389 species, representing a 44.5% of the total species number of the tribe (an increase of c. 137% compared with the 1097 reports gathered in the 1999 version). This increase is due both to chromosome research progress (analysed as counts/year) and an improved information capture system (including checking of populations location through Cyrillic alphabet, and Japanese and Chinese writing systems). Additionally, recent taxonomic advances, synonimization and new phylogenetic criteria have also been taken in account. The main basic number x = 8 is found at 2x, 3x, 4x, 5x, 6x, and 8x ploidy levels, whereas x = 9 is much rarer. -

Phylogenetic Insight Into Subgenera Idaeobatus and Malachobatus
Wang et al. Molecular Cytogenetics (2015) 8:11 DOI 10.1186/s13039-015-0114-y RESEARCH Open Access Phylogenetic insight into subgenera Idaeobatus and Malachobatus (Rubus, Rosaceae) inferring from ISH analysis Yan Wang1, Xiaorong Wang1,2*, Qing Chen1, Li Zhang1, Haoru Tang1, Ya Luo1 and Zejing Liu1 Abstract Background: Rubus is a large and taxonomically complex genus exhibiting agamospermy, polyploidy and frequent hybridization. The objective of this work was to elucidate rDNA disrtibution pattern and investigate genomic composition of polyploids in 16 Rubus taxa (2n =2x,3x,4x,8x) of two subgenera Idaeobatus and Malachobatus by ISH method. Results: The basic Rubus genome had one 45S rDNA locus, and all the polyploids (except R. setchuenensis) had the expected multiples of this number. Diploid and tetraploid Rubus taxa carried two 5S rDNA, whereas the triploid and octoploid species only had three. The duplicated 45S rDNA sites tended to be conserved, whereas those of 5S rDNA tended to be eliminated after polyploidization. The accession R03-20 was an autotriploid R. parvifolius, while R03-27 and R03-57 were naturally-occurred triploid hybrids between R. parvifolius and R. coreanus. GISH results suggested that R. parvifolius had close relationship with polyploids from Malachobatus. Conclusions: The polyploids from Malachobatus were probable allopolyploid. In addition, Rubus parvifolius might be involved in hybridization, polyploidization and speciation of some Idaeobatus and Malachobatus species. Keywords: Rubus, Allopolyploid, Hybrid, rDNA-FISH, GISH Background complex, with tetraploidy, hexaploidy, octoploidy or tetra- Rubus Linnaeus (Rosaceae) has long been deemed taxo- decaploidy level [6,11,12]. Interestingly, R. parvifolius in nomically challenging due to its propensity for agamo- subg. -

A Guide to Priority Plant and Animal Species in Oregon Forests
A GUIDE TO Priority Plant and Animal Species IN OREGON FORESTS A publication of the Oregon Forest Resources Institute Sponsors of the first animal and plant guidebooks included the Oregon Department of Forestry, the Oregon Department of Fish and Wildlife, the Oregon Biodiversity Information Center, Oregon State University and the Oregon State Implementation Committee, Sustainable Forestry Initiative. This update was made possible with help from the Northwest Habitat Institute, the Oregon Biodiversity Information Center, Institute for Natural Resources, Portland State University and Oregon State University. Acknowledgments: The Oregon Forest Resources Institute is grateful to the following contributors: Thomas O’Neil, Kathleen O’Neil, Malcolm Anderson and Jamie McFadden, Northwest Habitat Institute; the Integrated Habitat and Biodiversity Information System (IBIS), supported in part by the Northwest Power and Conservation Council and the Bonneville Power Administration under project #2003-072-00 and ESRI Conservation Program grants; Sue Vrilakas, Oregon Biodiversity Information Center, Institute for Natural Resources; and Dana Sanchez, Oregon State University, Mark Gourley, Starker Forests and Mike Rochelle, Weyerhaeuser Company. Edited by: Fran Cafferata Coe, Cafferata Consulting, LLC. Designed by: Sarah Craig, Word Jones © Copyright 2012 A Guide to Priority Plant and Animal Species in Oregon Forests Oregonians care about forest-dwelling wildlife and plants. This revised and updated publication is designed to assist forest landowners, land managers, students and educators in understanding how forests provide habitat for different wildlife and plant species. Keeping forestland in forestry is a great way to mitigate habitat loss resulting from development, mining and other non-forest uses. Through the use of specific forestry techniques, landowners can maintain, enhance and even create habitat for birds, mammals and amphibians while still managing lands for timber production. -

Mountain Plants of Northeastern Utah
MOUNTAIN PLANTS OF NORTHEASTERN UTAH Original booklet and drawings by Berniece A. Andersen and Arthur H. Holmgren Revised May 1996 HG 506 FOREWORD In the original printing, the purpose of this manual was to serve as a guide for students, amateur botanists and anyone interested in the wildflowers of a rather limited geographic area. The intent was to depict and describe over 400 common, conspicuous or beautiful species. In this revision we have tried to maintain the intent and integrity of the original. Scientific names have been updated in accordance with changes in taxonomic thought since the time of the first printing. Some changes have been incorporated in order to make the manual more user-friendly for the beginner. The species are now organized primarily by floral color. We hope that these changes serve to enhance the enjoyment and usefulness of this long-popular manual. We would also like to thank Larry A. Rupp, Extension Horticulture Specialist, for critical review of the draft and for the cover photo. Linda Allen, Assistant Curator, Intermountain Herbarium Donna H. Falkenborg, Extension Editor Utah State University Extension is an affirmative action/equal employment opportunity employer and educational organization. We offer our programs to persons regardless of race, color, national origin, sex, religion, age or disability. Issued in furtherance of Cooperative Extension work, Acts of May 8 and June 30, 1914, in cooperation with the U.S. Department of Agriculture, Robert L. Gilliland, Vice-President and Director, Cooperative Extension -

Restoration Objectives and Strategies for Terrestrial Habitats and Species of the Willamette Sub-Basin
Protection, Restoration, and Management of Terrestrial Habitats and Species of the Willamette Sub-Basin Technical Appendix 1 Contents 1. Introduction 1 1.1 Purpose and Objectives 1 1.2 Scope and Scale of the Report 2 1.3 Principal Sources of Data 5 1.4 Analytical Approaches 7 1.5 Building Upon Previous Efforts 11 1.6 How to Apply this Report and Databases to Decision-making 14 2. Focal Habitats and Associated Focal Species 31 2.1 Introduction 31 2.2 Focal Habitat: Oak Woodlands 37 2.2.1 Definition 37 2.2.2 Recognition of Importance 37 2.2.3 Status and Distribution 37 2.2.4 Past Impacts, Limiting Factors, and Future Threats 38 2.2.5 Protection, Restoration, and Management 39 2.2.6 Compatibility of Oak Woodland Management and Stream Habitat Management. 40 2.2.7 Contribution of Oak Woodlands to Regional Biodiversity 40 2.2.8 Selected Focal Species 40 2.2.9 Synthesis: Indicators of Oak Woodland Ecological Condition and Sustainability 53 2.3 Focal Habitat: Upland Prairie, Savanna, and Rock Outcrops 55 2.3.1 Description 55 2.3.2 Recognition of Importance 55 2.3.3 Status and Distribution 56 2.3.4 Past Impacts, Limiting Factors, and Future Threats 57 2.3.5 Protection, Restoration, and Management 58 2.3.6 Compatibility of Upland Prairie-Savanna Management and Stream Management 58 2.3.7 Contribution of Upland Prairie-Savanna to Regional Biodiversity 59 2.3.8 Selected Focal Species 59 2.3.9 Synthesis: Indicators of Ecological Condition and Sustainability for Upland Prairie- Savanna 77 2.4 Focal Habitat: Wetland Prairie and Seasonal Marsh 81 2.4.1 -
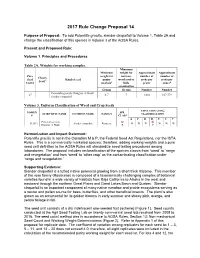
2017 Rule Change Proposal 14
2017 Rule Change Proposal 14 Purpose of Proposal: To add Potentilla gracilis, slender cinquefoil to Volume 1, Table 2A and change the classification of this species in Volume 3 of the AOSA Rules. Present and Proposed Rule: Volume 1. Principles and Procedures Table 2A. Weights for working samples. Minimum Minimum weight for Approximate Approximate Pure weight for noxious- number of number of Chaffy Seed Kind of seed purity weed seed or seeds per seeds per Seed a Unit # analysisb bulk gramc ounced examination Grams Grams Number Number Potentilla gracilis Douglas ex Hook 27 0.7 7 3,802 107,779 slender cinquefoil Volume 3. Uniform Classification of Weed and Crop Seeds CONTAMINATING NOMEN SPP. SCIENTIFIC NAME COMMON NAME FAMILY CLASSIFICATION # CLASS A F H R S T V Potentilla gracilis W W 313631 slender cinquefoil Rosaceae W W W W W W Douglas ex Hook R C Harmonization and Impact Statement: Potentilla gracilis is not in the Canadian M & P, the Federal Seed Act Regulations, nor the ISTA Rules. This is a commercially marketed species; therefore, adding working weights and a pure seed unit definition to the AOSA Rules will standardize seed testing procedures among laboratories. The proposal includes reclassification of the species classis from ‘weed’ to ‘range and revegetation’ and from ‘weed’ to ‘other crop’ as the contaminating classification under ‘range and revegetation.’ Supporting Evidence: Slender cinquefoil is a tufted native perennial growing from a short thick rhizome. This member of the rose family (Rosaceae) is composed of a taxonomically challenging complex of botanical varieties found in a wide variety of habitats from Baja California to Alaska in the west and eastward through the northern Great Plains and Great Lakes Basin and Quebec. -

Mckayla Stevens, Donald H. Mansfield James F. Smith Mary Ann E. Feist
RESOLVING THE ANOMALY OF LOMATIUM ANOMALUM: DISCOVERY OF A NEW SPECIES IN SOUTHWESTERN IDAHO (U.S.A.), LOMATIUM ANDRUSIANUM (APIACEAE) Mckayla Stevens, Donald H. Mansfield James F. Smith The College of Idaho Boise State University 2112 Cleveland Blvd. 1910 W. University Drive Caldwell, Idaho 83605, U.S.A. Boise, Idaho 83725, U.S.A. Mary Ann E. Feist University of Wisconsin-Madison 430 Lincoln Drive Madison, Wisconsin 53706, U.S.A. ABSTRACT Apparent polyphyly within the unresolved clade of Lomatium (Apiaceae) containing L. triternatum, L. anomalum, L. thompsonii, and L. pack- ardiae suggests conflict among current taxonomic classification schemes. To recover this clade and more clearly define species boundaries, we examined populations of L. anomalum from three geographic regions in Idaho and adjacent Oregon. Using phylogenetic, morphological, and ecological data, we conclude that the L. anomalum complex currently circumscribes multiple species. Phylogenetic analysis of the nuclear ribosomal ITS and ETS, and cpDNA rpl32-trnLUAG, rps-16 intron, trnD-trnT, ndhA intron, and psbA-trnH recovered populations from the Boise foothills as a distinct, monophyletic clade. Principal Components Analysis of 30 reproductive and vegetative characters show two distinct groups: one of Boise foothills and one of the combined Mann Creek and Camas Prairie vicinities. Principal Components Analysis of 16 soil characteristics show that soils occupied by Boise foothills populations are distinct from those occupied by Mann Creek and Camas Prairie populations. Based on phylogenetic, morphometric, and ecologic criteria, populations of what had been considered L. anomalum from the Boise foothills and vicinity are here described as a new species—Lomatium andrusianum. -

Baja California, Mexico, and a Vegetation Map of Colonet Mesa Alan B
Aliso: A Journal of Systematic and Evolutionary Botany Volume 29 | Issue 1 Article 4 2011 Plants of the Colonet Region, Baja California, Mexico, and a Vegetation Map of Colonet Mesa Alan B. Harper Terra Peninsular, Coronado, California Sula Vanderplank Rancho Santa Ana Botanic Garden, Claremont, California Mark Dodero Recon Environmental Inc., San Diego, California Sergio Mata Terra Peninsular, Coronado, California Jorge Ochoa Long Beach City College, Long Beach, California Follow this and additional works at: http://scholarship.claremont.edu/aliso Part of the Biodiversity Commons, Botany Commons, and the Ecology and Evolutionary Biology Commons Recommended Citation Harper, Alan B.; Vanderplank, Sula; Dodero, Mark; Mata, Sergio; and Ochoa, Jorge (2011) "Plants of the Colonet Region, Baja California, Mexico, and a Vegetation Map of Colonet Mesa," Aliso: A Journal of Systematic and Evolutionary Botany: Vol. 29: Iss. 1, Article 4. Available at: http://scholarship.claremont.edu/aliso/vol29/iss1/4 Aliso, 29(1), pp. 25–42 ’ 2011, Rancho Santa Ana Botanic Garden PLANTS OF THE COLONET REGION, BAJA CALIFORNIA, MEXICO, AND A VEGETATION MAPOF COLONET MESA ALAN B. HARPER,1 SULA VANDERPLANK,2 MARK DODERO,3 SERGIO MATA,1 AND JORGE OCHOA4 1Terra Peninsular, A.C., PMB 189003, Suite 88, Coronado, California 92178, USA ([email protected]); 2Rancho Santa Ana Botanic Garden, 1500 North College Avenue, Claremont, California 91711, USA; 3Recon Environmental Inc., 1927 Fifth Avenue, San Diego, California 92101, USA; 4Long Beach City College, 1305 East Pacific Coast Highway, Long Beach, California 90806, USA ABSTRACT The Colonet region is located at the southern end of the California Floristic Province, in an area known to have the highest plant diversity in Baja California. -

Native Herbaceous Plants in Our Gardens
Native Herbaceous Plants in Our Gardens A Guide for the Willamette Valley Native Gardening Awareness Program A Committee of the Emerald Chapter of the Native Plant Society of Oregon Members of the Native Gardening Awareness Program, a committee of the Emerald chapter of the NPSO, contributed text, editing, and photographs for this publication. They include: Mieko Aoki, John Coggins, Phyllis Fisher, Rachel Foster, Evelyn Hess, Heiko Koester, Cynthia Lafferty, Danna Lytjen, Bruce Newhouse, Nick Otting, and Michael Robert Spring 2005 1 2 Table of Contents Native Herbaceous Plants in Our Gardens ...........................5 Shady Woodlands .................................................................7 Baneberry – Actaea rubra ................................................... 7 Broad-leaved Bluebells – Mertensia platyphylla .....................7 Hound’s-tongue – Cynoglossum grande ............................... 8 Broad-leaved Starflower –Trientalis latifolia ..........................8 Bunchberry – Cornus unalaschkensis (formerly C. canadensis) ....8 False Solomon’s-seal – Maianthemum racemosum ................ 9 Fawn Lily – Erythronium oregonum .........................................9 Ferns ..........................................................................10-12 Fringecup – Tellima grandiflora and T. odorata ..................... 12 Inside-out Flower – Vancouveria hexandra ....................... 13 Large-leaved Avens – Geum macrophyllum ...................... 13 Meadowrue – Thalictrum spp. ...............................................14