EMS Protocols
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

2016 Recognition Targets for Hospitals
Patient Safety Reporting Program 2016 Recognition Targets for Hospitals March 2016 Recognition targets provide attainable goals for healthcare facilities participating in the Patient Safety Reporting Program (PSRP). Targets can also help facilities incrementally and effectively build adverse event review and reporting into their culture of safety. On a statewide level, targets ensure OPSC receives enough adverse event reports to build a strong database of prevention strategies so that all Oregon healthcare facilities can learn from one another. Targets are effective as of January 1, 2016. Although a participant may meet or exceed targets at any time throughout the year, the facility should continue to report adverse events according to reporting program guidelines. Healthcare facilities that meet targets are recognized annually for their transparency efforts and commitment to patient safety. For more information, visit oregonpatientsafety.org. Meet Targets Exceed Targets Quantity The number of reports submitted by a participating Submit at least the Submit at least the facility minimum quantity minimum quantity target for target for Goal: Help ensure PSRP obtains enough reports to your facility your facility build a strong database for learning. (see page 2) (see page 2) Acceptable Quality The report identifies contributing factors, root Submit at least 2 Submit 75% of causes, and system-level action plans reports that are reports with acceptable quality acceptable quality Goal: Provide enough information so that your experience can help others learn and improve. See the Guide to Quality Reporting Timeliness The amount of time that passes between discovery Submit 50% of reports of an adverse event and when a report is submitted within 45 days of to PSRP event discovery Goal: Respond immediately after an adverse event to collect full and reliable information, reduce delays, and develop strong solutions. -

Weekly Report
COVID-19 Weekly Report Oregon’s Weekly Surveillance Summary Novel Coronavirus (COVID-19) Weekly Data Report - February 3, 2021 Table of Contents Background ........................................................................................................................................... 4 Weekly Report Summary ..................................................................................................................... 5 Clinical characteristics and risk factors ............................................................................................... 6 Epidemiologic links ........................................................................................................................... 6 Figure 1. Epidemiologic link of recent COVID-19 cases by week of onset ............................... 7 Figure 2. Epidemiologic link of all COVID-19 cases by week of onset ...................................... 8 Figure 3. Recent sporadic COVID-19 cases by race and week of onset .................................... 9 Figure 4. All sporadic COVD-19 cases by race and week of onset .......................................... 10 Figure 5. Recent sporadic COVID-19 cases by ethnicity and week of onset .......................... 11 Figure 6. All sporadic COVID-19 cases by ethnicity and week of onset .................................. 12 Clinical symptoms and reported risk factors ................................................................................ 12 Figure 7. Reported signs and symptoms for all COVID-19 cases ........................................... -
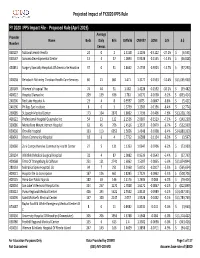
FY 2020 Proposed Rule Impact File.Xlsx
Projected Impact of FY2020 IPPS Rule FY 2020 IPPS Impact File ‐ Proposed Rule (April 2019) Average Provider Name Beds Daily Bills CMIV36 CMIV37 ∆CMI ∆ % ∆ $ Number Census 060107 National Jewish Health 24 0 2 1.5158 1.1036 ‐0.4122 ‐27.2%$ (6,595) 050547 Sonoma Developmental Center 13 4 37 1.0893 0.9338 ‐0.1555 ‐14.3% $ (46,028) 450831 Surgery Specialty Hospitals Of America Se Houston 37 0 31 2.8662 2.4739 ‐0.3923 ‐13.7%$ (97,290) 320038 Rehoboth Mckinley Christian Health Care Services 60 21 861 1.471 1.3177 ‐0.1533 ‐10.4%$ (1,055,930) 150149 Women's Hospital The 74 44 31 1.582 1.4228 ‐0.1592 ‐10.1%$ (39,482) 400022 Hospital Damas Inc 209 139 636 1.781 1.6172 ‐0.1638 ‐9.2%$ (833,414) 240206 Red Lake Hospital A 23 4 8 0.9597 0.875 ‐0.0847 ‐8.8%$ (5,421) 240196 Phillips Eye Institute 8 0 3 1.3739 1.2583 ‐0.1156 ‐8.4%$ (2,774) 260085 St Joseph Medical Center 173 104 2870 1.8602 1.7196 ‐0.1406 ‐7.6%$ (3,228,176) 400122 Professional Hospital Guaynabo Inc 54 13 122 2.1536 2.0007 ‐0.1529 ‐7.1%$ (149,230) 330086 Montefiore Mount Vernon Hospital 63 45 706 1.4516 1.3537 ‐0.0979 ‐6.7%$ (552,939) 050030 Oroville Hospital 133 113 6052 1.5656 1.4648 ‐0.1008 ‐6.4%$ (4,880,333) 460043 Orem Community Hospital 18 6 4 1.7712 1.6588 ‐0.1124 ‐6.3%$ (3,597) 320060 Zuni Comprehensive Community Health Center 27 5 131 1.1353 1.0647 ‐0.0706 ‐6.2%$ (73,989) 250134 Whitfield Medical Surgical Hospital 32 4 87 1.0082 0.9539 ‐0.0543 ‐5.4%$ (37,793) 420068 Trmc Of Orangeburg & Calhoun 251 121 2741 1.6062 1.5207 ‐0.0855 ‐5.3%$ (1,874,844) 280133 Nebraska Spine Hospital, -

Hospital Presumptive Medical -- Authorized Sites 1-Nov-14
Hospital Presumptive Medical -- Authorized Sites 1-Nov-14 Hospital Identification Number Contact Email Phone Adventist Medical Center Portland 1801887658 Donna Krenzler [email protected] 503.251.6153 Asante Tonya Richner [email protected] 541-789-5208 Asante Ashland Community Hospital 1386644029; 136114 Asante Three Rivers Medical Center 1801891809; 022560 Asante Rogue Regional Medical Center 1770587107; 162008 Blue Mountain Hospital District 1356414395 Barb Oliver [email protected] 541-575-4153 Good Shepherd Medical Center 1295789667 Jonathan Edwards [email protected] 541-667-3438 Grande Ronde Hospital 1407446195; 067801 KARLI WRIGHT [email protected] 541-963-1555 Kaiser Permanente Northwest 1124182902 Melissa Inskeep [email protected] 503 813 4111 Lake Health District 1376698522 Brian McCarthy [email protected] 541-947-7290 Legacy System Terrie Handy [email protected] Melody Jackson [email protected] Kim Carter [email protected] Legacy Emanuel 1831112358 Legacy Good Samaritan 1780608216 Legacy Meridian Park 1184647620 Legacy Mt. Hood 1255354700 Legacy Randall Children's Hospital at Legacy Emanuel 1831112358 Legacy Salmon Creek 1700809829 Lower Umpqua Hospital 1003874819; 000054; 286986 Mark Sayler [email protected] 541-271-2171 x395 Mercy Medical Center 1477590198 John Kasberger Kasberger [email protected] Luke Martin [email protected] 303-513-2502 Laurie Jones [email protected] 541-677-2461 OHSU Hospital 1609824010 Kippi Coffey [email protected] 503-494-6664 PeaceHealth -

ROR) + Library Partnership
WLA/OLA 2019 – Charting the Unknown Together Healthcare Providers & Libraries Collaborating to Support Families How to start a Reach Out and Read (ROR) + Library Partnership Step 1: Check with your librarians, early learning staff, and Youth Services/Community Managers to see if relationships already exist. Talk internally to see what type/level of partnership might make sense. Step 2: Find out if a ROR clinic exists near you! Washington https://reachoutandreadwa.org/ - 206.524.3579 - [email protected] Oregon: http://reachoutandread.org/oregon/ - 503-830-0510 - [email protected] Find a program near you: http://www.reachoutandread.org/resource-center/find-a-program/ For large scale partnerships, identify the best person at your library to reach out to the ROR director for your state. For local, clinic-to-branch level connections, ROR staff can help with making an initial introduction. In some cases, there may be value in setting up an in-person meeting with ROR, the clinic, and the library. In others, an email introduction may be ideal. Step 3: Get to know your clinic! Set up a phone or in-person meeting. Here are some questions you might ask: I am interested in knowing more about the communities you serve at your clinic. Do you serve low income families or families from specific cultural groups? What languages are spoken as a first language by your clients? What community resources do you frequently refer families to? Do you have any questions for me about what resources are available at the library? WLA/OLA -

Oregon Health Plan (Medicaid), Medicare and Commercial Members
2016 Provider Workshop Presented by Moda Health Agenda • Moda Networks • OEBB & PEBB Plan Changes • Credentialing • Primary Care Support • Prior Authorizations updates • Ensuring Accurate Claims • Risk Adjustment • Contacting Moda Health Moda Networks Total membership 266,403 42,269 46,763 Commercial Medicare Medicaid - EOCCO Northern Coast membership 10,165 3,971 Commercial Medicare Southern Coast membership 8,191 1,328 Commercial Medicare Southern Valley membership 22,478 4,527 Commercial Medicare Eastern Oregon membership 46,763 19,108 3,745 Commercial Medicare Medicaid - EOCCO Portland Metro membership 92,245 9,878 Commercial Medicare The Gorge membership 4,194 827 Commercial Medicare Central Oregon membership 21,943 2,660 Commercial Medicare Willamette Valley membership 37,891 4,649 Commercial Medicare West Central Valley membership 32,935 6,776 Commercial Medicare 2017 Network overview Affinity (Individual plans) Medicare Advantage Beacon (Individual plans) EOCCO (Medicaid) Connexus (Large and small group employer plans) Summit Synergy (Large and small group (Large and small group employer plans) employer plans) 2017 Individual Network lineup Clackamas, Clatsop, Columbia, Coos, Curry, Beacon Hood River, Jackson, Josephine, Marion, Select Western Oregon Multnomah, Polk, Wasco, counties Washington and Yamhill counties Affinity Baker, Grant, Gilliam, Harney, Lake, Malheur, Eastern Oregon Morrow, Sherman, counties Umatilla, Union, Wallowa and Wheeler counties Beacon Network Effective January 1, 2017 What is different? • Smaller geography • Clinically integrated network, which includes nine health system partners and their referring providers • Simplified plan design options (1 bronze, 3 silver and 3 gold) • PCP selection is required Beacon Network 1. Adventist Health 4 2. Asante 6 3. Bay Area Hospital 8 1 5 4. -

Medical Facility Directory
NORTHWEST REGION PUBLISHED OCtober 2020 MEDICAL FACILITY DIRECTORY YOUR GUIDE TO CONNECTING TO CARE This directory applies to the following networks: Classic, Classic Complete, Classic + Dental Choice, Added Choice, Added Choice + Dental Choice GO GREEN! View or download the Medical Facility Directory at a time that is convenient for you. Go to kp.org/directory/nw. If you would like a printed copy of our Participating and Select Provider Directory, please call Member Services at 1-800-813-2000 to request a copy be sent to your home. To report provider directory inaccuracies, please call Member Services at 1-800-813-2000 or go to kp.org/webmanager COVID-19 Update: To help ensure the safety of our members, staff, and community, we may need to temporarily close or limit in-person services at some of our medical and dental facilities. You can still get the care you need, including in-person emergency care, e-visits, phone or video visits, and more. For the most up to date information please visit kp.org/coronavirus. kp.org/directory/nw All plans offered and underwritten by Kaiser Foundation Health Plan of the Northwest. 19411_10-20 500 NE Multnomah St., Suite 100, Portland, OR 97232. PROVIDER SELECTION CRITERIA Kaiser Foundation Health Plan of the Northwest (KFHPNW) offers several networks that are comprised of the Kaiser Permanente integrated health care delivery system. The primary contracted provider groups and facilities that participate include: Northwest Permanente, P.C., Physicians and Surgeons, facilities owned and operated by Kaiser Foundation Hospitals (hospitals, Kaiser Permanente medical offices, Kaiser Permanente pharmacies), The Portland Clinic, The CHP Group, and contracted hospitals. -
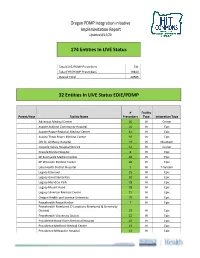
Oregon PDMP Integration Initiative Implementation Report Updated 8/12/20
Oregon PDMP Integration initiative Implementation Report Updated 8/12/20 174 Entities In LIVE Status Total EDIE/PDMP Prescribers 741 Total EHR/PDMP Prescribers 19844 Overall Total 20585 32 Entities In LIVE Status EDIE/PDMP # Facility Parent/Host Facility Name Prescribers Type Integration Type Adventist Medical Center 26 IH Cerner Asante Ashland Community Hospital 10 IH Epic Asante Rogue Regional Medical Center 41 IH Epic Asante Three Rivers Medical Center 19 IH Epic CHI St. Anthony Hospital 19 IH Meditech Coquille Valley Hospital District 12 IH Cerner Grande Ronde Hospital 8 IH Epic KP Sunnyside Medical Center 36 IH Epic KP Westside Medical Center 36 IH Epic Lake Health District Hospital 5 IH T-System Legacy Emanuel 25 IH Epic Legacy Good Samaritan 30 IH Epic Legacy Meridian Park 18 IH Epic Legacy Mount Hood 28 IH Epic Legacy Silverton Medical Center 15 IH Epic Oregon Health and Science University 70 IH Epic Peacehealth Peace Harbor 7 IH Epic Peacehealth Riverbend (2 Locations Riverbend & University District) 22 IH Epic Peacehealth University District 22 IH Epic Providence Hood River Memorial Hospital 10 IH Epic Providence Medford Medical Center 19 IH Epic Providence Milwaukie Hospital 13 IH Epic # Facility Parent/Host Facility Name Prescribers Type Integration Type Providence Newberg Hospital 26 IH Epic Providence Portland Medical Center 43 IH Epic Providence Seaside Hospital 7 IH Epic Providence St Vincent Medical Center 47 IH Epic Providence Willamette Falls Medical Center 12 IH Epic Salem Hospital 42 IH Epic Sky Lakes Medical Center -
Name of CCO County SBHC in Counties Primary CCO Applicant Participants of CCO Contact Info Baker Baker HS GOBHI GOBHI
Name of CCO County SBHC in Counties Primary CCO Applicant Participants of CCO Contact Info Baker Baker HS GOBHI GOBHI Benton-Linn -Lincoln Lincoln ES, Monroe Linn and Benton county govts. ES, Newport HS, Taft Benton County Health Department Mitch Anderson (541) 766-6805 (Health MS/HS, Toledo HS, Director Walport HS Samaritan Health (IHC) Intercommunity Health Network (FQHC) Accountable Behavioral Health Alliance Mid-Valley Behavioral Care Network Corvallis Clinic Cascade West council of Governments Clatsop no SBHC GOBHI GOBHI Clatsop County govt. Coastal Family Health Center (FQHC) Jim Coffee Columbia Memorial Hospital Providence Seaside Hospital Clatsop Behavioral Health Care Family Care Care Oregon Clatsop Care Lower Columbia IPA Clatsop Medical Society NW Senior and Disability Services Sunset Empire Transportation District Dr. Tom Duncan Columbia Lewis & Clack GOBHI Coastal Family Health Center (FQHC) Campus, and Rainer Community health groups? K-12 Coos-Curry Marchfield Bay Area Hospital HS,Powers SD, Docs ? Brooking Harbor HS Others? Central Oregon Deschutes-Crook- Crook River ES, Community Health Alliance, 1813 W. Harvard, Health Council Jefferson Ensworth SBHC, La Suite 448, Roseburg, Oregon 97471, Phone: Pine K-12, Lynch, 541.440.4491 SBHC, Redmond HS, Sisters HS Central Oregon Deschutes-Crook- Crook River ES, Health Council Jefferson Ensworth SBHC, La Pine K-12, Lynch, SBHC, Redmond HS, Deschutes-Crook-Jefferson govts. Sisters HS Tammy Baney- County Commissioner (541) 388- 6567 email:[email protected] St. Charles Health System Jim diegel (541) 706-5849 Maryclair Jorgensen-Director Contracting email: [email protected] Health Matters (behavioral health) Mosaic Medical Clinic Megan Haase (541) 617-5372 PacificSource Dan Stevens- VP Network Development (541) 330-8101 email: [email protected] Community Douglas Douglas HS, Douglas Co. -

COVID-19 Weekly Report
COVID-19 Weekly Report Oregon’s Weekly Surveillance Summary Novel Coronavirus (COVID-19) Weekly Data Report - September 29, 2021 Table of Contents Background ..................................................................................................................................... 3 Weekly Report Summary ................................................................................................................ 4 Clinical Characteristics and risk factors .......................................................................................... 5 Epidemiologic links ..................................................................................................................... 5 Figure 1. Epidemiologic link of recent COVID-19 cases by week of onset ............................. 6 Figure 2. Epidemiologic link of all COVID-19 cases by week of onset .................................... 7 Figure 3. Recent sporadic COVID-19 cases by race and week of onset .................................. 8 Figure 4. All sporadic COVD-19 cases by race and week of onset .......................................... 9 Figure 5. Recent sporadic COVID-19 cases by ethnicity and week of onset ........................ 10 Figure 6. All sporadic COVID-19 cases by ethnicity and week of onset ................................ 11 Clinical symptoms and reported risk factors ............................................................................ 11 Figure 7. Reported signs and symptoms for all COVID-19 cases ......................................... -

Hospital Presumptive Medical -- Authorized Sites
Hospital Presumptive Medical -- Authorized Sites Hospital Identification Number Contact Email Phone Adventist Medical Center Portland 1801887658 Donna Krenzler [email protected] 503.251.6153 Blue Mountain Hospital District 1356414395 Barb Oliver [email protected] 541-575-4153 Good Shepherd Medical Center 1295789667 Jonathan Edwards [email protected] 541-667-3438 Grande Ronde Hospital 1407446195; 067801 KARLI WRIGHT [email protected] 541-963-1555 Kaiser Permanente Northwest 1124182902 Melissa Inskeep [email protected] 503 813 4111 Legacy System Terrie Handy [email protected] Melody Jackson [email protected] Kim Carter [email protected] Legacy Emanuel 1831112358 Legacy Good Samaritan 1780608216 Legacy Meridian Park 1184647620 Legacy Mt. Hood 1255354700 Legacy Randall Children's Hospital at Legacy Emanuel 1831112358 Legacy Salmon Creek 1700809829 Lower Umpqua Hospital 1003874819; 000054; 286986 Mark Sayler [email protected] 541-271-2171 x395 Mercy Medical Center 1477590198 John Kasberger [email protected] Luke Martin [email protected] 303-513-2502 Laurie Jones [email protected] 541-677-2461 OHSU Hospital 1609824010 Kippi Coffey [email protected] 503-494-6664 Providence Health & Services Lesa Ellis [email protected] 503-513-3930 Providence Hood River Memorial Hospital 1255429338 Providence Medford Medical Center 1689755670 Providence Milwaukie Hospital 1366536963 Providence Newberg Medical Center 1952482275 Providence Portland Medical Center 1003971845 Providence Seaside Hospital -

Regional Pediatric Specialty Care Capacity Report
Table of Contents I. Report Introduction ...................................................................... 1 II. Report Process & Methods............................................................ 2 III. Inpatient Unit Data A. Emergency Department................................................ 3 B. Acute Pediatric Units..................................................... 4 C. Neonatal Intensive Care Unit (NICU)..............................5 D. Pediatric Intensive Care Unit (PICU).............................. 6 IV. Hospital Based Support Units A. Post Anesthesia Care Unit (PACU)................................. 7 B. Respiratory Therapy...................................................... 7 C. Surgical Services........................................................... 8 V. Provider Pool................................................................................ 9 VI. Outpatient Services...................................................................... 12 VII. Pediatric Services Expansion Plans............................................... 12 VIII. Telemedicine Services................................................................... 13 IX. Strengths, Challenges, & Planning Opportunities......................... 17 X. Acknowledgments........................................................................ 19 I. Report Introduction The Pediatric Surge Leadership Group (PSLG) is a chartered Work Group of the NW Oregon Health Preparedness Organization (HPO), the regional Health Care Coalition (HCC) serving emergency planning