Bruising, Petechia, Ecchymosis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Dermatologic Manifestations and Complications of COVID-19
American Journal of Emergency Medicine 38 (2020) 1715–1721 Contents lists available at ScienceDirect American Journal of Emergency Medicine journal homepage: www.elsevier.com/locate/ajem Dermatologic manifestations and complications of COVID-19 Michael Gottlieb, MD a,⁎,BritLong,MDb a Department of Emergency Medicine, Rush University Medical Center, United States of America b Department of Emergency Medicine, Brooke Army Medical Center, United States of America article info abstract Article history: The novel coronavirus disease of 2019 (COVID-19) is associated with significant morbidity and mortality. While Received 9 May 2020 much of the focus has been on the cardiac and pulmonary complications, there are several important dermato- Accepted 3 June 2020 logic components that clinicians must be aware of. Available online xxxx Objective: This brief report summarizes the dermatologic manifestations and complications associated with COVID-19 with an emphasis on Emergency Medicine clinicians. Keywords: COVID-19 Discussion: Dermatologic manifestations of COVID-19 are increasingly recognized within the literature. The pri- fi SARS-CoV-2 mary etiologies include vasculitis versus direct viral involvement. There are several types of skin ndings de- Coronavirus scribed in association with COVID-19. These include maculopapular rashes, urticaria, vesicles, petechiae, Dermatology purpura, chilblains, livedo racemosa, and distal limb ischemia. While most of these dermatologic findings are Skin self-resolving, they can help increase one's suspicion for COVID-19. Emergency medicine Conclusion: It is important to be aware of the dermatologic manifestations and complications of COVID-19. Knowledge of the components is important to help identify potential COVID-19 patients and properly treat complications. © 2020 Elsevier Inc. -

Skin Injuries – Can We Determine Timing and Mechanism?
Skin injuries – can we determine timing and mechanism? Jo Tully VFPMS Seminar 2016 What skin injuries do we need to consider? • Bruising • Commonest accidental and inflicted skin injury • Basic principles that can be applied when formulating opinion • Abrasions • Lacerations }we need to be able to tell the difference • Incisions • Stabs/chops • Bite marks – animal v human / inflicted v ‘accidental’ v self-inflicted Our role…. We are often/usually/always asked…………….. • “What type of injury is it?” • “When did this injury occur?” • “How did this injury occur?” • “Was this injury inflicted or accidental?” • IS THIS CHILD ABUSE? • To be able to answer these questions (if we can) we need knowledge of • Anatomy/physiology/healing - injury interpretation • Forces • Mechanisms in relation to development, plausibility • Current evidence Bruising – can we really tell which bruises are caused by abuse? Definitions – bruising • BLUNT FORCE TRAUMA • Bruise =bleeding beneath intact skin due to BFT • Contusion = bruise in deeper tissues • Haematoma - extravasated blood filling a cavity (or potential space). Usually associated with swelling • Petechiae =Pinpoint sized (0.1-2mm) hemorrhages into the skin due to acute rise in venous pressure • medical causes • direct forces • indirect forces Medical Direct Indirect causes mechanical mechanical forces forces Factors affecting development and appearance of a bruise • Properties of impacting object or surface • Force of impact • Duration of impact • Site - properties of body region impacted (blood supply, -
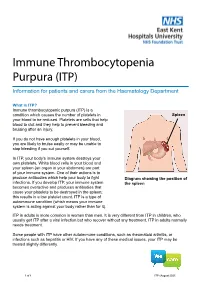
Immune Thrombocytopenia Purpura (ITP)
Immune Thrombocytopenia Purpura (ITP) Information for patients and carers from the Haematology Department What is ITP? Immune thrombocytopenic purpura (ITP) is a condition which causes the number of platelets in Spleen your blood to be reduced. Platelets are cells that help blood to clot and they help to prevent bleeding and bruising after an injury. If you do not have enough platelets in your blood, you are likely to bruise easily or may be unable to stop bleeding if you cut yourself. In ITP, your body’s immune system destroys your own platelets. White blood cells in your blood and your spleen (an organ in your abdomen) are part of your immune system. One of their actions is to produce antibodies which help your body to fight Diagram showing the position of infections. If you develop ITP, your immune system the spleen becomes overactive and produces antibodies that cause your platelets to be destroyed in the spleen; this results in a low platelet count. ITP is a type of autoimmune condition (which means your immune system is acting against your body rather than for it). ITP in adults is more common in women than men. It is very different from ITP in children, who usually get ITP after a viral infection but who recover without any treatment. ITP in adults normally needs treatment. Some people with ITP have other autoimmune conditions, such as rheumatoid arthritis, or infections such as hepatitis or HIV. If you have any of these medical issues, your ITP may be treated slightly differently. 1 of 8 ITP (August 2021) A normal platelet count is between 150 and 400 thousand million platelets per litre of blood. -

A Rare Syndrome with Alemtuzumab, Review of Monitoring Protocol
Open Access Case Report DOI: 10.7759/cureus.5715 Idiopathic Thrombocytopenic Purpura: A Rare Syndrome with Alemtuzumab, Review of Monitoring Protocol Deepika Sarvepalli 1 , Mamoon Ur Rashid 2 , Waqas Ullah 3 , Yousaf Zafar 4 , Muzammil Khan 5 1. Internal Medicine, Guntur Medical College, Guntur, IND 2. Internal Medicine, AdventHealth, Orlando, USA 3. Internal Medicine, Abington Hospital - Jefferson Health, Abington, USA 4. Internal Medicine, University of Missouri - Kansas City School of Medicine, Kansas City, USA 5. Internal Medicine, Khyber Teaching Hospital, Peshawar, PAK Corresponding author: Deepika Sarvepalli, [email protected] Abstract Alemtuzumab, a humanized monoclonal antibody that targets surface molecule CD52, causes rapid and complete depletion of circulating T- and B-lymphocytes through antibody-dependent cell-mediated and complement-mediated cytotoxicity. Alemtuzumab has demonstrated superior efficacy compared to subcutaneous interferon beta-1a (SC IFNB-1a) in patients with multiple sclerosis (MS). Alemtuzumab treatment causes a rare and distinct form of secondary immune thrombocytopenic purpura (ITP), characterized by delayed onset, responsiveness to conventional therapies, and prolonged remission following treatment. In phase two and three clinical trials, the incidence of ITP was higher with alemtuzumab treatment compared to the patients receiving SC IFNB-1a. Here we report a case of ITP occurring two years after the first treatment with alemtuzumab. The patient recovered completely after a timely diagnosis -

Bleeds and Bruises in Children with Haemophilia
Bleeds and Bruises in CHildren WiTH HaeMOPHilia MusCle ANd/or JoiNt Bleeds Call the parent/guardian P.r.i.C.e. siGNs oF A serious HeAd Bleed P : Protection * Headache. Lower Limb: Take weight off the joint or muscle * drowsiness. Upper Limb: No carrying using affected arm * Nausea. r : rest * Vomiting. • Rest means rest! * unsteady Balance. • Try not to allow use of the joint or muscle where * irritability. possible. * Confusion. * seizures. i : ice * loss of consciousness. • Regular ice packs can help with pain & reduce swelling. • Put an ice pack over the affected area for 20 minutes. Repeat every two hours. DO NOT leave the ice pack on for more than 20 minutes siGNs oF A soFt tissue DO NOT place ice pack directly on skin (Use a tea Bleed towel/cold pack cover) * Bruising, discolouring of skin. C : Compression * Mild swelling. • Use an elasticated bandage to compress the affected area to reduce swelling. e : elevation • Elevate the affected limb to help reduce swelling. siGNs oF AN ABdoMiNAl • Keep the affected joint or muscle above the level of the Bleed heart. * Bloody, black or tar-like First Aid bowel motions. * red or brown urine. Mouth & Gum Bleeds * Pain. These can be hard to control because clots that form are * Vomiting of blood (blood washed away by saliva or knocked off by the tongue or food. Try giving the child an ice cube or ice pop to suck. may be red or black). These bleeds may need treatment by parents or the treatment centre. Nosebleeds siGNs oF BleediNG iNto tHe Tilt head forward and pinch the bridge of the nose below the bone for 10 - 20 minutes and / or put an ice-pack on JoiNts or MusCles the bridge of the nose for not more than 5 minutes. -

Isolated Plantar Vein Thrombosis Resembling a Corn with a Bruise
JE Hahm, et al pISSN 1013-9087ㆍeISSN 2005-3894 Ann Dermatol Vol. 31, No. 1, 2019 https://doi.org/10.5021/ad.2019.31.1.66 CASE REPORT Isolated Plantar Vein Thrombosis Resembling a Corn with a Bruise Ji Eun Hahm, Kang Su Kim, Jae Won Ha, Chul Woo Kim, Sang Seok Kim Department of Dermatology, Kangdong Sacred Heart Hospital, College of Medicine, Hallym University, Seoul, Korea Plantar vein thrombosis, rarely-reported disease, is usually or callus, plantar fibromatosis, or plantar verruca1. Among accompanied by pain and tenderness in the plantar region laborers, they may develop from excess pressure on the and should be differentiated from other dermatological con- bony prominences of the feet, repetitive uneven friction ditions causing plantar pain, such as hemorrhagic corn/cal- from footwear, or gait abnormalities. Plantar vein throm- lus, plantar epidermal cyst, verruca, or plantar fibromatosis. bosis is a rare condition causing plantar pain. The exact A 52-year-old man presented with a violaceous tender sub- cause of plantar vein thrombosis is yet unclear, but predis- cutaneous nodule overlying a hyperkeratotic plaque on his posing conditions, such as prior trauma, surgery, paraneo- sole. Initially, he thought it was a corn and applied keratolytic plastic syndromes, or coagulation disorders have been agents, which failed to work. Sonography revealed a well-de- described. To date, there is no established treatment ex- marcated mass with increased peripheral vascularity. His cept surgical excision, but reportedly, nonsteroidal anti-in- pain was relieved after a complete wide excision, which con- flammatory drug or heparin with elastic bandage is known firmed the mass to be plantar vein thrombosis after histo- to be effective for symptomatic control2-5. -

Bruise, Contusion & Ecchymosis Conventions
Bruise, Contusion and Ecchymosis MedDRA Proactivity Proposal Implementation MedDRA Version 16.0 I. MSSO Recognized Definitions of Concepts and Terms The MSSO has designated Dorland’s Illustrated Medical Dictionary as the standard reference for medical definitions. The following definitions are cited from Dorland’s 27th edition: Bruise – A superficial injury produced by impact without laceration; a contusion Contusion – A bruise; an injury of a part without a break in the skin Ecchymosis – A small hemorrhagic spot, larger than a petechia, in the skin or mucous membrane forming a nonelevated, rounded or irregular, blue or purplish patch. Hematoma – A localized collection of blood, usually clotted, in an organ, space, or tissue, due to a break in the wall of a blood vessel. Hemorrhage – The escape of blood from the vessels; bleeding. Petechia – A pinpoint, non-raised, perfectly round, purplish red spot caused by intradermal or submucous hemorrhage. Additional comments regarding the definitions: Bruise and contusion are synonymous, and are often used in a colloquial context. Bruise and contusion are each considered a result of injury. Bruise and contusion have been used to describe minor hemorrhage within tissue, where traumatized blood vessels leak blood into the interstitial space. Commonly, capillaries and sometimes venules are injured within skin, subcutaneous tissue, muscle, or bone. In addition to trauma, the terms bruise, ecchymosis, and to a lesser extent, contusion, have also been used as clinical signs of disorders of platelet function, coagulopathies, venous congestion, allergic reactions, etc. Hemorrhage may be used to describe blood escaping from vessels and retained in the interstitial space, and perhaps more commonly, to describe the escape of blood from vessels, and flowing freely external to the tissues. -

Understanding Haemophilia
Understanding haemophilia Understanding haemophilia Contents Introduction 3 Haemophilia and your child 4 What is haemophilia? 5 What causes haemophilia? 5 Can females have haemophilia? 6 Carriers 8 Who is affected by haemophilia? 9 How severe is haemophilia? 9 Signs and symptoms of haemophilia 11 How is haemophilia diagnosed? 14 Diagnosis 14 Treatment 16 Port-a-cath 19 Managing joint bleeds with PRICE 19 Gene therapy 21 Possible complications of haemophilia 22 Inhibitors 22 Joint damage 22 Medical and dental treatment 23 Surgery Circumcision Dental care Medicines Vaccinations Bleeding disorder card Living with haemophilia 26 Sport and exercise 27 School, college and work 28 Travel 29 Pregnancy and haemophilia 30 Glossary of terms 32 About The Haemophilia Society 33 2 Understanding haemophilia Introduction This booklet is about haemophilia A and B. It gives a general overview of haemophilia and information on diagnosing, treating and living with the condition that we hope will answer your main questions. It has been written for people directly affected by haemophilia and for anyone interested in learning about haemophilia. If you are a parent and your child has recently been diagnosed with haemophilia you may be feeling quite overwhelmed. Remember, you’re not alone and many families are facing the same concerns and issues. Please do get in touch – we have lots of support and information available as well as services for parents and children. You can find out more via our website or Facebook pages, by emailing [email protected] or calling us on 020 7939 0780. The outlook is now the best it has ever been for people with haemophilia in the UK. -

EFFIENT Safely and Effectively
1 HIGHLIGHTS OF PRESCRIBING INFORMATION • Patients with unstable angina or, non-ST-elevation myocardial These highlights do not include all the information needed to use infarction (NSTEMI) (1.1). EFFIENT safely and effectively. See full prescribing information • Patients with ST-elevation myocardial infarction (STEMI) when for EFFIENT. managed with either primary or delayed PCI (1.1). EFFIENT (prasugrel) tablets, for oral use ------------------------ DOSAGE AND ADMINISTRATION ----------------------- Initial U.S. Approval: 2009 • Initiate treatment with a single 60-mg oral loading dose (2). • Continue at 10-mg once daily with or without food. Consider 5-mg WARNING: BLEEDING RISK once daily for patients <60 kg (2). See full prescribing information for complete boxed warning. • Patients should also take aspirin (75-mg to 325-mg) daily (2). • Effient can cause significant, sometimes fatal, bleeding (5.1, ---------------------- DOSAGE FORMS AND STRENGTHS --------------------- 5.2, 6.1). • Do not use Effient in patients with active pathological bleeding 5-mg and 10-mg tablets (3) or a history of transient ischemic attack or stroke (4.1, 4.2). ------------------------------- CONTRAINDICATIONS ------------------------------ • In patients ≥75 years of age, Effient is generally not • Active pathological bleeding (4.1) recommended, except in high-risk patients (diabetes or prior • Prior transient ischemic attack or stroke (4.2) MI), where its use may be considered (8.5). • Hypersensitivity to prasugrel or any component of the product • Do not start Effient in patients likely to undergo urgent (4.3) coronary artery bypass graft surgery (CABG). When possible, discontinue Effient at least 7 days prior to any surgery (5.2). ------------------------ WARNINGS AND PRECAUTIONS ----------------------- • Additional risk factors for bleeding include: body weight • CABG-related bleeding: Risk increases in patients receiving <60 kg; propensity to bleed; concomitant use of medications Effient who undergo CABG (5.2). -

Approach to Bleeding Diathesi
Approach to Bleeding Diathesis Dr.Nalini K Pati MD, DNB, DCH (Syd), FRCPA Paediatric Haematologist Royal Children’s Hospital Melbourne Australia Objectives Objectives - I I. Clinical aspects of bleeding Clinical aspects of bleeding II. Hematologic disorders causing bleeding • Coagulation factor disorders • Platelet disorders III. Approach to acquired bleeding disorders • Hemostasis in liver disease • Surgical patients • Warfarin toxicity IV. Approach to laboratory abnormalities • Diagnosis and management of thrombocytopenia V. Drugs and blood products used for bleeding Clinical Features of Bleeding Disorders Petechiae Platelet Coagulation (typical of platelet disorders) disorders factor disorders Site of bleeding Skin Deep in soft tissues Mucous membranes (joints, muscles) (epistaxis, gum, vaginal, GI tract) Petechiae Yes No Ecchymoses (“bruises”) Small, superficial Large, deep Hemarthrosis / muscle bleeding Extremely rare Common Do not blanch with pressure Bleeding after cuts & scratches Yes No (cf. angiomas) Bleeding after surgery or trauma Immediate, Delayed (1-2 days), usually mild often severe Not palpable (cf. vasculitis) Ecchymoses (typical of coagulation factor disorders) Objectives - II Hematologic disorders causing bleeding – Coagulation factor disorders – Platelet disorders Coagulation factor disorders Hemophilia A and B Inherited bleeding Acquired bleeding Hemophilia A Hemophilia B disorders disorders Coagulation factor deficiency Factor VIII Factor IX – Hemophilia A and B – Liver disease – vonWillebrands disease – Vitamin K Inheritance X-linked X-linked recessive recessive – Other factor deficiencies deficiency/warfarin overdose Incidence 1/10,000 males 1/50,000 males –DIC Severity Related to factor level <1% - Severe - spontaneous bleeding 1-5% - Moderate - bleeding with mild injury 5-25% - Mild - bleeding with surgery or trauma Complications Soft tissue bleeding Hemarthrosis (acute) Hemophilia Clinical manifestations (hemophilia A & B are indistinguishable) Hemarthrosis (most common) Fixed joints Soft tissue hematomas (e. -

VAXZEVRIA/COVID-19 Vaccine Astrazeneca: Risk of Thrombosis in Combination with Thrombocytopenia – Updated Information
AstraZeneca Block B Liffey Valley Office Campus Dublin 22 D22 X0Y3 astrazeneca.com 2nd June 2021 VAXZEVRIA/COVID-19 Vaccine AstraZeneca: Risk of thrombosis in combination with thrombocytopenia – Updated information Dear Healthcare Professional, Please refer to previous Direct Healthcare Professional Communications (DHPCs) of 24th March and 13th April, 2021. AstraZeneca AB in agreement with the European Medicines Agency and the Health Products Regulatory Authority (HPRA) would like to inform you of the following: Summary Vaxzevria is contraindicated in individuals who have experienced Thrombosis with Thrombocytopenia Syndrome (TTS) following previous vaccination with Vaxzevria. TTS requires specialised clinical management. Healthcare professionals should consult applicable guidance and/or consult specialists (e.g., haematologists, specialists in coagulation) to diagnose and treat this condition. Individuals diagnosed with thrombocytopenia within 3 weeks after vaccination with Vaxzevria should be actively investigated for signs of thrombosis. Similarly, individuals who present with thrombosis within 3 weeks of vaccination should be evaluated for thrombocytopenia. The Vaxzevria Summary of Product Characteristics (SmPC) has been updated accordingly with this information. Background on the safety concern Vaxzevria is indicated for active immunisation to prevent COVID-19 caused by SARS-CoV-2, in individuals 18 years of age and older. A combination of thrombosis and thrombocytopenia, in some cases accompanied by bleeding, has been observed very rarely following vaccination with Vaxzevria. This includes severe cases presenting as venous thrombosis, including in unusual sites, such as cerebral venous sinus thrombosis and splanchnic vein thrombosis, as well as arterial thrombosis, concomitant with AstraZeneca Pharmaceuticals (Ireland) DAC T: +353 1 609 7100 F: +353 1 679 6650 Registered Office: 6th Floor, South Bank House, Barrow Street, Dublin 4, D04 TR29, Ireland Registered in Ireland No. -

Blood Clot 13 .Indd
MECHANICAL DEVICES PLEASE NOTE: GRADUATED COMPRESSION STOCKINGS (GCS) How long should I keep using the anticoagu- lant drug? These are tight stockings that squeeze your lower legs. They help keep the blood fl owing through the What should I do if I can’t give myself the shot? veins in your legs. Your legs will be measured to If you have any questions about the drugs used REDUCING fi t the stockings properly. Wear them all the time, to prevent a clot, please ask your doctor, nurse except when washing or showering until you re- or pharmacist. Your Risk of turn to your normal level of daily activity. Let your If you were given graduated compression doctor or nurse know if your skin changes color or BLOOD CLOTS stockings, they should be worn until you return if you develop blisters or pain from the stockings. to your usual level of activity. OR VTES And make sure the stockings don’t roll down to the space behind your kneecap. If you develop any symptoms of possible Deep Vein Thrombosis or Pulmonary Embolus at SEQUENTIAL COMPRESSION DEVICES (SCD) home in the days and weeks after hospitaliza- These are infl atable sleeves that are placed on tion, then seek medical advice immediately, your legs. They will squeeze your legs on and off either from your doctor or your nearest hospital during the day. Take them off before you get out of emergency department. bed because they can cause you to trip and fall. Reproduced with permission from Hotel-Dieu Be sure to ask for the sleeves to be put back on.