Plastid Phylogenomic Insights Into the Evolution of the Caprifoliaceae S.L. (Dipsacales)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Toward a Resolution of Campanulid Phylogeny, with Special Reference to the Placement of Dipsacales
TAXON 57 (1) • February 2008: 53–65 Winkworth & al. • Campanulid phylogeny MOLECULAR PHYLOGENETICS Toward a resolution of Campanulid phylogeny, with special reference to the placement of Dipsacales Richard C. Winkworth1,2, Johannes Lundberg3 & Michael J. Donoghue4 1 Departamento de Botânica, Instituto de Biociências, Universidade de São Paulo, Caixa Postal 11461–CEP 05422-970, São Paulo, SP, Brazil. [email protected] (author for correspondence) 2 Current address: School of Biology, Chemistry, and Environmental Sciences, University of the South Pacific, Private Bag, Laucala Campus, Suva, Fiji 3 Department of Phanerogamic Botany, The Swedish Museum of Natural History, Box 50007, 104 05 Stockholm, Sweden 4 Department of Ecology & Evolutionary Biology and Peabody Museum of Natural History, Yale University, P.O. Box 208106, New Haven, Connecticut 06520-8106, U.S.A. Broad-scale phylogenetic analyses of the angiosperms and of the Asteridae have failed to confidently resolve relationships among the major lineages of the campanulid Asteridae (i.e., the euasterid II of APG II, 2003). To address this problem we assembled presently available sequences for a core set of 50 taxa, representing the diver- sity of the four largest lineages (Apiales, Aquifoliales, Asterales, Dipsacales) as well as the smaller “unplaced” groups (e.g., Bruniaceae, Paracryphiaceae, Columelliaceae). We constructed four data matrices for phylogenetic analysis: a chloroplast coding matrix (atpB, matK, ndhF, rbcL), a chloroplast non-coding matrix (rps16 intron, trnT-F region, trnV-atpE IGS), a combined chloroplast dataset (all seven chloroplast regions), and a combined genome matrix (seven chloroplast regions plus 18S and 26S rDNA). Bayesian analyses of these datasets using mixed substitution models produced often well-resolved and supported trees. -

Lonicera Spp
Species: Lonicera spp. http://www.fs.fed.us/database/feis/plants/shrub/lonspp/all.html SPECIES: Lonicera spp. Choose from the following categories of information. Introductory Distribution and occurrence Botanical and ecological characteristics Fire ecology Fire effects Fire case studies Management considerations References INTRODUCTORY SPECIES: Lonicera spp. AUTHORSHIP AND CITATION FEIS ABBREVIATION SYNONYMS NRCS PLANT CODE COMMON NAMES TAXONOMY LIFE FORM FEDERAL LEGAL STATUS OTHER STATUS AUTHORSHIP AND CITATION: Munger, Gregory T. 2005. Lonicera spp. In: Fire Effects Information System, [Online]. U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station, Fire Sciences Laboratory (Producer). Available: http://www.fs.fed.us/database/feis/ [2007, September 24]. FEIS ABBREVIATIONS: LONSPP LONFRA LONMAA LONMOR LONTAT LONXYL LONBEL SYNONYMS: None NRCS PLANT CODES [172]: LOFR LOMA6 LOMO2 LOTA LOXY 1 of 67 9/24/2007 4:44 PM Species: Lonicera spp. http://www.fs.fed.us/database/feis/plants/shrub/lonspp/all.html LOBE COMMON NAMES: winter honeysuckle Amur honeysuckle Morrow's honeysuckle Tatarian honeysuckle European fly honeysuckle Bell's honeysuckle TAXONOMY: The currently accepted genus name for honeysuckle is Lonicera L. (Caprifoliaceae) [18,36,54,59,82,83,93,133,161,189,190,191,197]. This report summarizes information on 5 species and 1 hybrid of Lonicera: Lonicera fragrantissima Lindl. & Paxt. [36,82,83,133,191] winter honeysuckle Lonicera maackii Maxim. [18,27,36,54,59,82,83,131,137,186] Amur honeysuckle Lonicera morrowii A. Gray [18,39,54,60,83,161,186,189,190,197] Morrow's honeysuckle Lonicera tatarica L. [18,38,39,54,59,60,82,83,92,93,157,161,186,190,191] Tatarian honeysuckle Lonicera xylosteum L. -

Outline of Angiosperm Phylogeny
Outline of angiosperm phylogeny: orders, families, and representative genera with emphasis on Oregon native plants Priscilla Spears December 2013 The following listing gives an introduction to the phylogenetic classification of the flowering plants that has emerged in recent decades, and which is based on nucleic acid sequences as well as morphological and developmental data. This listing emphasizes temperate families of the Northern Hemisphere and is meant as an overview with examples of Oregon native plants. It includes many exotic genera that are grown in Oregon as ornamentals plus other plants of interest worldwide. The genera that are Oregon natives are printed in a blue font. Genera that are exotics are shown in black, however genera in blue may also contain non-native species. Names separated by a slash are alternatives or else the nomenclature is in flux. When several genera have the same common name, the names are separated by commas. The order of the family names is from the linear listing of families in the APG III report. For further information, see the references on the last page. Basal Angiosperms (ANITA grade) Amborellales Amborellaceae, sole family, the earliest branch of flowering plants, a shrub native to New Caledonia – Amborella Nymphaeales Hydatellaceae – aquatics from Australasia, previously classified as a grass Cabombaceae (water shield – Brasenia, fanwort – Cabomba) Nymphaeaceae (water lilies – Nymphaea; pond lilies – Nuphar) Austrobaileyales Schisandraceae (wild sarsaparilla, star vine – Schisandra; Japanese -

Heptacodium Miconioides
photograph © Peter Del Tredici, Arnold Arboretum of Harvard University An old multi-stemmed specimen of Heptacodium miconioides growing in woodland on Tian Tai Shan, Zhejiang, September 2004, probably the first wild plant ofHeptacodium to be seen by a Western botanist since its discovery by Ernest Wilson in 1907. Heptacodium miconioides Rehder In his last ‘Tree of the Year’, JOHN GRIMSHAW discusses what distinguishes a shrub from a tree and writes about a large shrub introduced to cultivation first in the early twentieth century and again in the late twentieth century, that is rare in the wild. Introduction When the terms of reference for New Trees were drawn up (see pp. 1-2), having agreed that the book would contain only trees, we had to address the deceptive question ‘what is a tree?’ Most of us ‘can recognise a tree when we see one’ and in many ways this is as good an answer as one needs, but when is a woody plant not a tree? When it’s a shrub, of course – but what is a shrub? In the end we adopted the simple definitions that a tree ‘normally [has] a single stem reaching or exceeding 5 m in height, at least in its native habitat. A shrub would normally have multiple woody stems emerging from the base or close to the ground, and would seldom exceed 5 m in height’ (Grimshaw & Bayton 2009). In most cases this served to make a clear distinction, but one of the casualties that fell between the two stools was the Chinese plant Heptacodium miconioides, a significant recent introduction described by theFlora of China (Yang et al. -

The Role of Species Including in Valerianella Genus in Phytocenoses in the Area of Nakhchivan Autonomous Republic // Бюллетень Науки И Практики
Бюллетень науки и практики / Bulletin of Science and Practice Т. 6. №7. 2020 https://www.bulletennauki.com https://doi.org/10.33619/2414-2948/56 UDC 635.57 https://doi.org/10.33619/2414-2948/56/04 AGRIS F40 THE ROLE OF SPECIES INCLUDING IN VALERIANELLA L. GENUS IN PHYTOCENOSES IN THE AREA OF NAKHCHIVAN AUTONOMOUS REPUBLIC ©Talibov T., Academician of Azerbaijan NAS, Dr. habil., Institute of Bioresources Nakhchivan branch of Azerbaijan NAS, Nakhchivan, Azerbaijan, [email protected] ©Mahmudova U., ORCID: 0000-0003-2877-3761, Nakhchivan State University, Nakhchivan, Azerbaijan, [email protected] РОЛЬ ВИДОВ РОДА VALERIANELLA L., ВХОДЯЩИХ В НЕКОТОРЫЕ ФИТОЦЕНОЗЫ НАХИЧЕВАНСКОЙ АВТОНОМНОЙ РЕСПУБЛИКИ ©Талыбов Т. Г., акад. НАН Азербайджана, д-р биол. наук, Институт биоресурсов Нахичеванского отделения НАН Азербайджана, г. Нахичевань, Азербайджан, [email protected] ©Махмудова У. М., ORCID: 0000-0003-2877-3761, Нахичеванский государственный университет, г. Нахичевань, Азербайджан, [email protected] Abstract. In this article the structure of species including in Valerianella Genus was studied with geobotanical methods, their role in phytocenosis, their formations, and associations were determined in the vegetation cover in Soyugdagh and Khorhat mountainous areas of Ordubad region. It has been determined that although the species of the Valerianella Genus are small numbers in phytocenosis and associations, they have great importance in improving the fodder quality of the vegetation. This plays an important role in the enrichment of food ration of Bezoar Goat and other herbivorous animals that inhabit the area of Zangezur National Park. Аннотация. В данной статье изучена структура видов рода Valerianella геоботаническими методами, определена их роль в фитоценозе, определены их формации и ассоциации в растительном покрове горных районов Союгдаг и Хорхат Ордубадского района. -

Phylogeny and Phylogenetic Taxonomy of Dipsacales, with Special Reference to Sinadoxa and Tetradoxa (Adoxaceae)
PHYLOGENY AND PHYLOGENETIC TAXONOMY OF DIPSACALES, WITH SPECIAL REFERENCE TO SINADOXA AND TETRADOXA (ADOXACEAE) MICHAEL J. DONOGHUE,1 TORSTEN ERIKSSON,2 PATRICK A. REEVES,3 AND RICHARD G. OLMSTEAD 3 Abstract. To further clarify phylogenetic relationships within Dipsacales,we analyzed new and previously pub- lished rbcL sequences, alone and in combination with morphological data. We also examined relationships within Adoxaceae using rbcL and nuclear ribosomal internal transcribed spacer (ITS) sequences. We conclude from these analyses that Dipsacales comprise two major lineages:Adoxaceae and Caprifoliaceae (sensu Judd et al.,1994), which both contain elements of traditional Caprifoliaceae.Within Adoxaceae, the following relation- ships are strongly supported: (Viburnum (Sambucus (Sinadoxa (Tetradoxa, Adoxa)))). Combined analyses of C ap ri foliaceae yield the fo l l ow i n g : ( C ap ri folieae (Diervilleae (Linnaeeae (Morinaceae (Dipsacaceae (Triplostegia,Valerianaceae)))))). On the basis of these results we provide phylogenetic definitions for the names of several major clades. Within Adoxaceae, Adoxina refers to the clade including Sinadoxa, Tetradoxa, and Adoxa.This lineage is marked by herbaceous habit, reduction in the number of perianth parts,nectaries of mul- ticellular hairs on the perianth,and bifid stamens. The clade including Morinaceae,Valerianaceae, Triplostegia, and Dipsacaceae is here named Valerina. Probable synapomorphies include herbaceousness,presence of an epi- calyx (lost or modified in Valerianaceae), reduced endosperm,and distinctive chemistry, including production of monoterpenoids. The clade containing Valerina plus Linnaeeae we name Linnina. This lineage is distinguished by reduction to four (or fewer) stamens, by abortion of two of the three carpels,and possibly by supernumerary inflorescences bracts. Keywords: Adoxaceae, Caprifoliaceae, Dipsacales, ITS, morphological characters, phylogeny, phylogenetic taxonomy, phylogenetic nomenclature, rbcL, Sinadoxa, Tetradoxa. -

PRE Evaluation Report for Lonicera Fragrantissima
PRE Evaluation Report -- Lonicera fragrantissima Plant Risk Evaluator -- PRE™ Evaluation Report Lonicera fragrantissima -- Georgia 2017 Farm Bill PRE Project PRE Score: 15 -- Evaluate this plant further Confidence: 65 / 100 Questions answered: 19 of 20 -- Valid (80% or more questions answered) Privacy: Public Status: Submitted Evaluation Date: November 13, 2017 This PDF was created on August 13, 2018 Page 1/18 PRE Evaluation Report -- Lonicera fragrantissima Plant Evaluated Lonicera fragrantissima Image by Kurt Stüber Page 2/18 PRE Evaluation Report -- Lonicera fragrantissima Evaluation Overview A PRE™ screener conducted a literature review for this plant (Lonicera fragrantissima) in an effort to understand the invasive history, reproductive strategies, and the impact, if any, on the region's native plants and animals. This research reflects the data available at the time this evaluation was conducted. Summary Sweet breath of spring (Lonicera fragrantissima) is a deciduous shrub that grows up to 6-10' tall and wide. It bears many small, white, and very fragrant flowers in the early spring, followed by small red berries that grow in early to mid summer. L. fragrantissima is naturalized across much of the Eastern U.S. and is listed on the Georgia and South Carolina EPPC sites as well as by the Tennessee Invasive Plant Council. General Information Status: Submitted Screener: Lila Uzzell Evaluation Date: November 13, 2017 Plant Information Plant: Lonicera fragrantissima Regional Information Region Name: Georgia Climate Matching Map To answer four of the PRE questions for a regional evaluation, a climate map with three climate data layers (Precipitation, UN EcoZones, and Plant Hardiness) is needed. These maps were built using a toolkit created in collaboration with GreenInfo Network, USDA, PlantRight, California-Invasive Plant Council, and The Information Center for the Environment at UC Davis. -

Part I Chinese Plant Names Index 2010-2017
This Book is Sponsored by Shanghai Chenshan Botanical Garden 上海辰山植物园 Shanghai Chenshan Plant Science Research Center, Chinese Academy of Sciences 中国科学院上海辰山植物科学研究中心 Special Fund for Scientific Research of Shanghai Landscaping & City Appearance Administrative Bureau (G182415) 上海市绿化和市容管理局科研专项 (G182415) National Specimen Information Infrastructure, 2018 Special Funds 中国国家标本平台 2018 年度专项 Shanghai Sailing Program (14YF1413800) 上海市青年科技英才扬帆计划 (14YF1413800) Chinese Plant Names Index 2010-2017 DU Cheng & MA Jin-shuang Chinese Plant Names Index 2010-2017 中国植物名称索引 2010-2017 DU Cheng & MA Jin-shuang Abstract The first two volumes of Chinese Plant Names Index (CPNI) cover the years 2000 through 2009, with entries 1 through 5,516, and 2010 through 2017, with entries 5,517 through 10,795. A unique entry is generated for the specific name of each taxon in a specific publication. Taxonomic treatments cover all novelties at the rank of family, genus, species, subspecies, variety, form and named hybrid taxa, new name changes (new combinations and new names), new records, new synonyms and new typifications for vascular plants reported or recorded from China. Detailed information on the place of publication, including author, publication name, year of publication, volume, issue, and page number, are given in detail. Type specimens and collects information for the taxa and their distribution in China, as well as worldwide, are also provided. The bibliographies were compiled from 182 journals and 138 monographs or books published worldwide. In addition, more than 400 herbaria preserve type specimens of Chinese plants are also listed as an appendix. This book can be used as a basic material for Chinese vascular plant taxonomy, and as a reference for researchers in biodiversity research, environmental protection, forestry and medicinal botany. -

The Influence of Environmental Variations on the Phenolic Compound Profiles and Antioxidant Activity of Two Medicinal Patagonian Valerians (Valeriana Carnosa Sm
AIMS Agriculture and Food, 6(1): 106–124. DOI: 10.3934/agrfood.2021007 Received: 11 November 2020 Accepted: 11 December 2020 Published: 15 December 2020 http://www.aimspress.com/journal/agriculture Research article The influence of environmental variations on the phenolic compound profiles and antioxidant activity of two medicinal Patagonian valerians (Valeriana carnosa Sm. and V. clarionifolia Phil.) Nicolas Nagahama1,2,3,*, Bruno Gastaldi2,3, Michael N. Clifford4, María M. Manifesto5 and Renée H. Fortunato2,5 1 Estación Experimental Agroforestal Esquel, Instituto Nacional de Tecnología Agropecuaria (INTA), Esquel, Chubut, Argentina 2 Consejo Nacional de Investigaciones Científicas y Tecnológicas (CONICET) 3 Laboratorio de Investigación de Plantas Aromáticas y Medicinales Nativas (LIPAM), FCNyCS, Universidad Nacional de la Patagonia San Juan Bosco, Esquel, Chubut, Argentina 4 School of Bioscience and Medicine, University of Surrey, Guildford, United Kingdom and Department of Nutrition, Dietetics and Food, Monash University, Notting Hill, Victoria, Australia 5 Instituto de Recursos Biológicos, CIRN- INTA, Hurlingham, Buenos Aires, Argentina * Correspondence: Email: [email protected]; Tel: (+54) 2945451558. Abstract: Valeriana carnosa and V. clarionifolia stand out as principal elements in the indigenous pharmacopeias of Patagonia; however, their phytochemical characterization is unknown. This study constitutes the starting point of a general project that aims to characterize secondary metabolites in these species. The variability of phenolic compounds in root ethanolic extracts was analyzed and compared for thirteen populations of V. carnosa and two of V. clarionifolia from the south of Argentinean Patagonia. Phenolic content was quantified by the Folin-Ciocalteu method and the putative phenolic compound profiles were investigated using HPLC-UV-MS. -
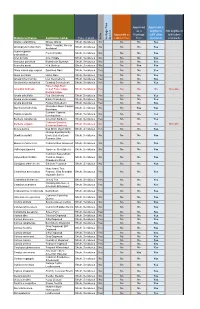
Botanical Name Common Name
Approved Approved & as a eligible to Not eligible to Approved as Frontage fulfill other fulfill other Type of plant a Street Tree Tree standards standards Heritage Tree Tree Heritage Species Botanical Name Common name Native Abelia x grandiflora Glossy Abelia Shrub, Deciduous No No No Yes White Forsytha; Korean Abeliophyllum distichum Shrub, Deciduous No No No Yes Abelialeaf Acanthropanax Fiveleaf Aralia Shrub, Deciduous No No No Yes sieboldianus Acer ginnala Amur Maple Shrub, Deciduous No No No Yes Aesculus parviflora Bottlebrush Buckeye Shrub, Deciduous No No No Yes Aesculus pavia Red Buckeye Shrub, Deciduous No No Yes Yes Alnus incana ssp. rugosa Speckled Alder Shrub, Deciduous Yes No No Yes Alnus serrulata Hazel Alder Shrub, Deciduous Yes No No Yes Amelanchier humilis Low Serviceberry Shrub, Deciduous Yes No No Yes Amelanchier stolonifera Running Serviceberry Shrub, Deciduous Yes No No Yes False Indigo Bush; Amorpha fruticosa Desert False Indigo; Shrub, Deciduous Yes No No No Not eligible Bastard Indigo Aronia arbutifolia Red Chokeberry Shrub, Deciduous Yes No No Yes Aronia melanocarpa Black Chokeberry Shrub, Deciduous Yes No No Yes Aronia prunifolia Purple Chokeberry Shrub, Deciduous Yes No No Yes Groundsel-Bush; Eastern Baccharis halimifolia Shrub, Deciduous No No Yes Yes Baccharis Summer Cypress; Bassia scoparia Shrub, Deciduous No No No Yes Burning-Bush Berberis canadensis American Barberry Shrub, Deciduous Yes No No Yes Common Barberry; Berberis vulgaris Shrub, Deciduous No No No No Not eligible European Barberry Betula pumila -

Vorläufiger Bericht Über Neuerungen in Der Im Entstehen Begriffenen Vierten Aufage Der Exkursionsfora
Neilreichia 9: 355–388 (2018) Vorläufiger Bericht über Neuerungen in der im Entstehen begriffenen vierten Auflage der Exkursionsflora Manfred A. Fischer1 & Peter Englmaier2 1 Department für Botanik und Biodiversitätsforschung, Universität Wien, Rennweg 14, 1030 Wien, Österreich; E-Mail: [email protected] 2 Fakultät für Lebenswissenschaften, Universität Wien, Althanstraße 14, 1090 Wien, Österreich, E-Mail: [email protected] Abstract: Provisional report on novelties approaching the fourth edition of the Austrian Excursion Flora Some geographic, taxonomic, floristic, and nomenclatural changes, for the time being, are re- ported. The geographical range is enlarged as to comprise the entire Eastern Alps, i. e. also the Bavarian Alps, eastern Grisons, the Italian Alps East of lake Como und the Slovenian Alps. In addition to changes mentioned already in Neilreichia 6 (2011) and 7 (2015), the most important changes are: Dryopteridaceae (s. lat.) are split into Athyriaceae, Cystopteridaceae, Dryopteri- daceae s. str., Onocleaceae and Woodsiaceae; Dryopteris lacunosa is a new apogamic species within D. affinis agg. – Aegilops and Triticum are kept separate; Bromus remains s. lat. (including Ceratochloa, Bromopsis, and Anisantha); Festuca includes Drymochloa, Leucopoa, Psilurus, and Vulpia; Lolium s. lat. includes Schedonorus; Koeleria is expanded to include Trisetum spicatum; Gaudinia and Trisetum s. str. remain separate; Pennisetum and Cenchrus are fused; Oloptum is split from Piptatherum; Poa and Bellardiochloa are separated; Psilathera and Sesleriella are split from Sesleria. – Lloydia is included in Gagea. – Neottia s. str. is maintained and Listera thus remains as a paraphyletic genus; Platanthera bifolia consists of two subspecies – Arenaria is split into Eremogone (E. -

SHRUBS & HEDGING ETC Abelia Grandiflora Evergreen Easy to Grow in Most Soil Conditions. Glossy Green Leaves with White Flowe
SHRUBS & HEDGING ETC Abelia grandiflora Evergreen Easy to grow in most soil conditions. Glossy green leaves with white flowers most of the year and reddish sepals to add more colour. Appreciates a yearly trim to stay tidy or to shape to your requirements. Abeliophyllum distichum White Forsythia White almond-scented flowers appear in early spring followed by glossy green foliage. Acca sellowiana Evergreen Pineapple Guava, Feijoa Fruit the size of an egg with pineapple/strawberry flavour. Tough evergreen shrub. Attractive flowers with bright red stamens. Dependable edible hedge. Agapanthus White & Blue Evergreen Long strap like leaves with tall stems holding the balls of bell-shaped flowers in blue or white. Arctotis Evergreen African Daisy Ground Cover Perennials with brightly coloured daisy-like flowers available in a wide range of colours. Artemisia absinthium Evergreen Wormwood 1 metre Europe and Nth Africa Attractive silver grey addition to the garden, benefits from annual pruning to keep growth thick. Has medicinal attributes….? Buddleja alternifolia Weeping Butterfly Bush Delicate mauve flowers packed in long clusters along drooping stems in spring and early summer. Buddleja colvelei Himalayan Butterfly Bush To 6 metres with large sprays of flowers up to 20 cm long in early summer. Buddleja davidii asstd colours Black Knight -almost black violet purple Gold –bright gold White –bright white Lochinch –pale violet Deciduous shrub requiring pruning once a year. Buxus harlandii Evergreen Harland’s Boxwood China Small narrow leaves, vase shaped growth. Cork like fissured bark, excellent for bonsai. Buxus microphylla Evergreen Japanese Box Faster growing so needs more trimming. Looser softer leaf. Buxus sempivirens Evergreen English Box Traditional small to medium hedging plant.