Bacterial Infection in the Pathogenesis of Variceal Bleeding. Is There Any Role for Antibiotic Prophylaxis in the Cirrhotic Patient?
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Clinical Audit on Management of Hematemesis in Children Admitted to Pediatric Gastroenterology and Hepatology Unit of Assiut
Med. J. Cairo Univ., Vol. 86, No. 8, December: 4531-4536, 2018 www.medicaljournalofcairouniversity.net Clinical Audit on Management of Hematemesis in Children Admitted to Pediatric Gastroenterology and Hepatology Unit of Assiut University Children Hospital ESRAA T. AHMED, M.Sc.; FATMA A. ALI, M.D. and NAGLA H. ABU FADDAN, M.D. The Department of Pediatrics, Faculty of Medicine, Assiut University, Assiut, Egypt Abstract Hematemesis: Indicates that the bleeding origin is above the Treitz angle, i.e., that it constitutes an Background: Hematemesis is an uncommon but potentially Upper Gastrointestinal Bleeding (UGIB) [3] . serious and life-threatening clinical condition in children. It indicates that the bleeding origin is above the Treitz angle, The etiology of upper GI bleeding varies by i.e., that it constitutes an Upper Gastrointestinal Bleeding (UGIB). age. The pathophysiology of upper GI bleeding is related to the source of the bleeding. Most clinically Aim of Study: To assess for how much the adopted proto- significant causes of upper GI bleeds are associated cols of management of children with upper gastrointestinal bleeding were applied at Gastroenterology & Hepatology Unit with ulcers, erosive esophagitis, gastritis, varices, of Assiut University Children Hospital. and/or Mallory-Weiss tears. While Physiologic Patients and Methods: This study is a an audit on man- stress, NSAIDs such as aspirin and ibuprofen, and agement of children with upper gastrointestinal bleeding infection with Helicobacter pylori are few of the admitted to pediatric Gastroenterology and Hepatology Unit, factors contributing to the imbalance leading to Assiut University Children Hospital during the period from ulcers and erosions in the GI tract [4] . -

Hemosuccus Pancreaticus: a Rare Cause of Upper Gastrointestinal Bleeding During Pregnancy Rani Akhil Bhat,1 Vani Ramkumar,1 K
Hemosuccus Pancreaticus: A Rare Cause Of Upper Gastrointestinal Bleeding During Pregnancy Rani Akhil Bhat,1 Vani Ramkumar,1 K. Akhil Krishnanand Bhat, 2 Rajgopal Shenoy2 Abstract Upper gastrointestinal bleeding is most commonly caused by From the 1Department of Department of Obstetrics and Gynaecology, Oman Medical 2 lesions in the esophagus, stomach or duodenum. Bleeding which College, Sohar, Sultanate of Oman, Department of Surgery, Oman Medical College, Sohar, Sultanate of Oma. originates from the pancreatic duct is known as hemosuccus pancreaticus. Only a few scattered case reports of hemosuccus Received: 06 Nov 2009 pancreaticus during pregnancy have been recorded in literature. Accepted: 31 Dec 2009 This is a case of a primigravida with 37 weeks of gestation Address correspondence and reprint request to: Dr. Rani A. Bhat,Department of with hemosuccus pancreaticus and silent chronic pancreatitis. Obstetrics and Gynaecology, Oman Medical College, P. O. Box 391, P. C. 321, Al- Evaluating pregnant women with upper gastrointestinal Tareef, Sohar, Sultanate of Oman. bleeding differs from that of non pregnant women as diagnostic E-mail: [email protected] modalities using radiation cannot be used. Therefore, Esophagogastroduodenoscopy should be performed at the time of active bleeding to diagnose hemosuccus pancreaticus. Bhat RA, et al. OMJ. 25 (2010); doi:10.5001/omj.2010.21 Introduction examination showed a combination of dark red blood and melena. Laboratory investigations revealed hemoglobin of 6.3 grams/dL, Hemosuccus pancreaticus is the term used to describe the liver function tests, serum amylase, glucose and prothrombin time syndrome of gastrointestinal bleeding into the pancreatic duct were within the normal range. -
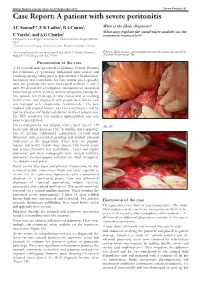
Case Report: a Patient with Severe Peritonitis
Malawi Medical Journal; 25(3): 86-87 September 2013 Severe Peritonitis 86 Case Report: A patient with severe peritonitis J C Samuel1*, E K Ludzu2, B A Cairns1, What is the likely diagnosis? 2 1 What may explain the small white nodules on the C Varela , and A G Charles transverse mesocolon? 1 Department of Surgery, University of North Carolina, Chapel Hill NC USA 2 Department of Surgery, Kamuzu Central Hospital, Lilongwe Malawi Corresponding author: [email protected] 4011 Burnett Womack Figure1. Intraoperative photograph showing the transverse mesolon Bldg CB 7228, Chapel Hill NC 27599 (1a) and the pancreas (1b). Presentation of the case A 42 year-old male presented to Kamuzu Central Hospital for evaluation of worsening abdominal pain, nausea and vomiting starting 3 days prior to presentation. On admission, his history was remarkable for four similar prior episodes over the previous five years that lasted between 3 and 5 days. He denied any constipation, obstipation or associated hematemesis, fevers, chills or urinary symptoms. During the first episode five years ago, he was evaluated at an outlying health centre and diagnosed with peptic ulcer disease and was managed with omeprazole intermittently . His past medical and surgical history was non contributory and he had no allergies and he denied alcohol intake or tobacco use. His HIV serostatus was negative approximately one year prior to presentation. On examination he was afebrile, with a heart rate of 120 (Fig 1B) beats/min, blood pressure 135/78 mmHg and respiratory rate of 22/min. Abdominal examination revealed mild distension with generalized guarding and marked rebound tenderness in the epigastrium. -

Esophageal Varices
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Crossref Hindawi Publishing Corporation Case Reports in Critical Care Volume 2016, Article ID 2370109, 4 pages http://dx.doi.org/10.1155/2016/2370109 Case Report A Rare but Reversible Cause of Hematemesis: (Downhill) Esophageal Varices Lam-Phuong Nguyen,1,2,3 Narin Sriratanaviriyakul,1,2,3 and Christian Sandrock1,2,3 1 Division of Pulmonary, Critical Care, and Sleep Medicine, University of California, Davis, Suite #3400, 4150 V Street, Sacramento, CA 95817, USA 2Department of Internal Medicine, University of California, Davis, Sacramento, USA 3VA Northern California Health Care System, Mather, USA Correspondence should be addressed to Lam-Phuong Nguyen; [email protected] Received 12 December 2015; Accepted 1 February 2016 Academic Editor: Kurt Lenz Copyright © 2016 Lam-Phuong Nguyen et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. “Downhill” varices are a rare cause of acute upper gastrointestinal bleeding and are generally due to obstruction of the superior vena cava (SVC). Often these cases of “downhill” varices are missed diagnoses as portal hypertension but fail to improve with medical treatment to reduce portal pressure. We report a similar case where recurrent variceal bleeding was initially diagnosed as portal hypertension but later found to have SVC thrombosis presenting with recurrent hematemesis. A 39-year-old female with history of end-stage renal disease presented with recurrent hematemesis. Esophagogastroduodenoscopy (EGD) revealed multiple varices. -

Hematemesis and Melena Chapter
126 CHAPTER 20 Hematemesis and melena Anthony Y. B. Teoh and James Y. W. Lau Chinese University of Hong Kong, Hong Kong SAR, China ESSENTIAL FACTS ABOUT CAUSATION ESSENTIALS OF TREATMENT Algorithm for management of acute GI bleeding Diagnosis Number of patients Mortality (%) 200716 (%) Major bleeding Minor bleeding Ulcer 1826 (27) 162 (8.9) (unstable hemodynamics) Erosive disease (gastric 1731 (26) 195 (14.1) Early elective upper and duodenum) Active resuscitation endoscopy Esophagitis 1177 (17) 65 (5.5) Urgent endoscopy Varices and portal 819 (12) 87 (14) Early administration of vasoactive hypertensive drugs in suspected variceal bleeding gastropathy Active ulcer bleeding Bleeding varices Malignancy 187 (3) 31 (17) Major stigmata Mallory-Weiss 213 (3) 10 (4.7) Endoscopic therapy Endoscopic therapy Adjunctive PPI Adjunctive vasoactive syndrome drugs Other diagnosis 797 (12) 125 (16) Success Failure Success Failure Continue Continue ulcer healing Recurrent Total 6750 675 (10) vasoactive drugs medications bleeding Variceal Data adapted from The United Kingdom National Audit in Upper Repeat endoscopic eradication Gastrointestinal Bleeding 2007 [16]. therapy program Sengstaken- Success Failure Blakemore tube ESSENTIALS OF DIAGNOSIS Angiographic embolization TIPS vs vs. surgery surgery • Symptoms: Coffee ground vomiting, hematemesis, melena, hematochezia, anemic symptoms • Past medical history: Liver cirrhosis, use of non-steroidal anti- inflammatory drugs • Signs: Hypotension, tachycardia, pallor, altered mental status, and therapeutic tool in managing these patients. Stratification melena or blood per rectum, decreased urine output of the patients into low- or high-risk groups aids in formulat- • Bloods: Anemia, raised urea, high urea to creatinine ratio • Endoscopy: Ulcers, varices, Mallory-Weiss tear, erosive disease, ing a clinical management plan and early endoscopy with neoplasms, vascular ectasia, and vascular malformations aggressive post-hemostasis care should be provided in high- risk patients. -

Esophageal Varices
World Gastroenterology Organisation Global Guidelines Esophageal varices JANUARY 2014 Revision authors Prof. D. LaBrecque (USA) Prof. A.G. Khan (Pakistan) Prof. S.K. Sarin (India) Drs. A.W. Le Mair (Netherlands) Original Review team Prof. D. LaBrecque (Chair, USA) Prof. P. Dite (Co-Chair, Czech Republic) Prof. Michael Fried (Switzerland) Prof. A. Gangl (Austria) Prof. A.G. Khan (Pakistan) Prof. D. Bjorkman (USA) Prof. R. Eliakim (Israel) Prof. R. Bektaeva (Kazakhstan) Prof. S.K. Sarin (India) Prof. S. Fedail (Sudan) Drs. J.H. Krabshuis (France) Drs. A.W. Le Mair (Netherlands) © World Gastroenterology Organisation, 2013 WGO Practice Guideline Esophageal Varices 2 Contents 1 INTRODUCTION ESOPHAGEAL VARICES............................................................. 2 1.1 WGO CASCADES – A RESOURCE -SENSITIVE APPROACH ............................................. 2 1.2 EPIDEMIOLOGY ............................................................................................................ 2 1.3 NATURAL HISTORY ...................................................................................................... 3 1.4 RISK FACTORS .............................................................................................................. 4 2 DIAGNOSIS AND DIFFERENTIAL DIAGNOSIS...................................................... 5 2.1 DIFFERENTIAL DIAGNOSIS OF ESOPHAGEAL VARICES /HEMORRHAGE ......................... 5 2.2 EXAMPLE FROM AFRICA — ESOPHAGEAL VARICES CAUSED BY SCHISTOSOMIASIS .. 6 2.3 OTHER CONSIDERATIONS ............................................................................................ -
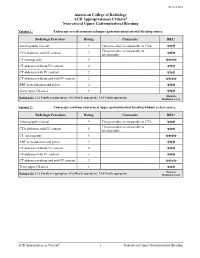
ACR Appropriateness Criteria® Nonvariceal Upper Gastrointestinal Bleeding
Revised 2016 American College of Radiology ACR Appropriateness Criteria® Nonvariceal Upper Gastrointestinal Bleeding Variant 1: Endoscopy reveals nonvariceal upper gastrointestinal arterial bleeding source. Radiologic Procedure Rating Comments RRL* Arteriography visceral 9 This procedure is comparable to CTA. ☢☢☢ This procedure is comparable to CTA abdomen with IV contrast 7 arteriography. ☢☢☢ CT enterography 5 ☢☢☢☢ CT abdomen without IV contrast 4 ☢☢☢ CT abdomen with IV contrast 2 ☢☢☢ CT abdomen without and with IV contrast 2 ☢☢☢☢ RBC scan abdomen and pelvis 2 ☢☢☢ X-ray upper GI series 1 ☢☢☢ *Relative Rating Scale: 1,2,3 Usually not appropriate; 4,5,6 May be appropriate; 7,8,9 Usually appropriate Radiation Level Variant 2: Endoscopy confirms nonvariceal upper gastrointestinal bleeding without a clear source. Radiologic Procedure Rating Comments RRL* Arteriography visceral 9 This procedure is comparable to CTA. ☢☢☢ This procedure is comparable to CTA abdomen with IV contrast 8 arteriography. ☢☢☢ CT enterography 5 ☢☢☢☢ RBC scan abdomen and pelvis 5 ☢☢☢ CT abdomen without IV contrast 4 ☢☢☢ CT abdomen with IV contrast 3 ☢☢☢ CT abdomen without and with IV contrast 3 ☢☢☢☢ X-ray upper GI series 1 ☢☢☢ *Relative Rating Scale: 1,2,3 Usually not appropriate; 4,5,6 May be appropriate; 7,8,9 Usually appropriate Radiation Level ACR Appropriateness Criteria® 1 Nonvariceal Upper Gastrointestinal Bleeding Variant 3: Nonvariceal upper gastrointestinal bleeding; negative endoscopy. Radiologic Procedure Rating Comments RRL* This procedure is comparable to CTA and Arteriography visceral 8 CT enterography. ☢☢☢ This procedure is comparable to CTA abdomen with IV contrast 8 arteriography and is an alternative to CT ☢☢☢ enterography. This procedure is comparable to CT enterography 7 arteriography and is an alternative to ☢☢☢☢ CTA. -

Hematemesis Melena Due to Helicobacter Pylori Infection in Duodenal Ulcer: a Case Report and Literature Review
International Journal of Science and Research (IJSR) ISSN (Online): 2319-7064 Index Copernicus Value (2016): 79.57 | Impact Factor (2017): 7.296 Hematemesis Melena due to Helicobacter Pylori Infection In Duodenal Ulcer: A Case Report and Literature Review Ayu Budhi Trisna Dewi Rahayu Sutanto1, I Made Suma Wirawan2 1General Practitioner Wangaya Hospital Denpasar Bali Indonesia 2 Endoscopy Unit of Internal Medicine Wangaya Hospital Denpasar Bali Indoensia Abstract: A Balinese woman, 60 years old complaint of hematemesis and melena. Esophagogastroduodenoscopy performed one day after admission and revealed a soliter ulcer at duodenum bulb. Histopathology examination revealed a spherical like organism suspected Helicobacter pylori (H. pylori) infection. Eradication of H. pylori by triple drug consisting of omeprazole, amoxicillin and chlarythromycin as the standard protocol of eradication within 14 days. Reevaluation by esophagogastroduodenoscopy examination will perform in the next 3 months to evaluate the treatment succesfull. Keywords: peptic ulcer, duodenum, H. pylori 1. Background also normal. The patient diagnosed with hematemesis suspect peptic ulcer. The patient was then admitted to ward Approximately 500,000 persons develop peptic ulcer disease and giving infusion ringer lactat, proton pump inhibitor in the United States each year. in 70 percent of patients it esomeprazole bolus 40 mg intravenous and continuous with occurs between the ages of 25 and 64 years. The annual 8 mg/ hours and planned for esofagogastroduodenoscopy to direct and indirect health care costs of the disease are evaluate the source of hematemesis. estimated at about $10 billion. However, the incidence of peptic ulcers is declining, possibly as a result of the increasing use of proton pump inhibitors and decreasing rates of Helicobacter pylori (H. -

Upper Gastrointestinal Tract Crohn's Disease
REVIEW Upper gastrointestinal tract Crohn's disease H UG H) FREEMAN. MD NY SITE WITHIN THI:: GASTROINTEST ABSTRACT: Crohn's disease may involve any site within the gastrointestinal inal tract may involved in Crohn's tract. Usually pathology is present in the ileum and/or colon, but atypical presen A be tations may occur with apparently 'isolated' involvement of the oropharynx, esoph disease (I) lt is well recognized that agus or gastroduodenum. lf changes typical of Crohn 's disease are detected in the Ueal and/or colonic involvement arc most upper gastrointestinal tract, then a careful assessment is required involving often present, but occasionally 'isolated' radiographic, endoscopic and histologic studies to determine if pathology is present disease locali zed to o ne or more sites in in more distal intestine. In addition, microbiologic studies may be important to the upper gastrointestinal tract occurs. exclude infectious causes, especially of granulomas. If these studies are negative, Indeed , if involvement with Crohn'sd1s prolonge; follow-up may be required to establish a diagnosis of Crohn's disease. ease is defined by the presence of subtle Although upper gastrointestinal involvement is increasingly recognized as a sig endoscopic changes including aph thoid nificant cause of morbidity in Crohn's disease, the treatment options arc limited, ulcera tions or histologic fe atures alone, largely anecdotal and need to be the subject of detailed cpidemiologic investiga rhe stomach and duodenum appear to tion and clinical trials. Can J Gastroenterol 1990;4(1):26-32 be relati vely common sites of Crohn's Key Words: Crohn 's disease, Inflammatory bowel disease , Gastrocolic fiscula . -

Diagnosis and Management of Autoimmune Hemolytic Anemia in Patients with Liver and Bowel Disorders
Journal of Clinical Medicine Review Diagnosis and Management of Autoimmune Hemolytic Anemia in Patients with Liver and Bowel Disorders Cristiana Bianco 1 , Elena Coluccio 1, Daniele Prati 1 and Luca Valenti 1,2,* 1 Department of Transfusion Medicine and Hematology, Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico, 20122 Milan, Italy; [email protected] (C.B.); [email protected] (E.C.); [email protected] (D.P.) 2 Department of Pathophysiology and Transplantation, Università degli Studi di Milano, 20122 Milan, Italy * Correspondence: [email protected]; Tel.: +39-02-50320278; Fax: +39-02-50320296 Abstract: Anemia is a common feature of liver and bowel diseases. Although the main causes of anemia in these conditions are represented by gastrointestinal bleeding and iron deficiency, autoimmune hemolytic anemia should be considered in the differential diagnosis. Due to the epidemiological association, autoimmune hemolytic anemia should particularly be suspected in patients affected by inflammatory and autoimmune diseases, such as autoimmune or acute viral hepatitis, primary biliary cholangitis, and inflammatory bowel disease. In the presence of biochemical indices of hemolysis, the direct antiglobulin test can detect the presence of warm or cold reacting antibodies, allowing for a prompt treatment. Drug-induced, immune-mediated hemolytic anemia should be ruled out. On the other hand, the choice of treatment should consider possible adverse events related to the underlying conditions. Given the adverse impact of anemia on clinical outcomes, maintaining a high clinical suspicion to reach a prompt diagnosis is the key to establishing an adequate treatment. Keywords: autoimmune hemolytic anemia; chronic liver disease; inflammatory bowel disease; Citation: Bianco, C.; Coluccio, E.; autoimmune disease; autoimmune hepatitis; primary biliary cholangitis; treatment; diagnosis Prati, D.; Valenti, L. -

Challenging Cases of Hospitalized Patients with Cirrhosis
Challenging cases of hospitalized patients with cirrhosis Danielle Brandman, MD, MAS Associate Professor of Clinical Medicine Program Director, Transplant Hepatology Fellowship Inpatient Chief of Service, Hepatology October 17, 2019 Disclosure ■ Grant/research support: Grifols Case 1 ■ 63M with HCV cirrhosis is hospitalized due to worsened fluid retention, with ascites and lower extremity edema Case 1 ■ 63M with HCV cirrhosis is hospitalized due to worsened fluid retention, with ascites and lower extremity edema ■ He denies fever or frank abdominal pain, though is uncomfortable from abdominal distension. Case 1 ■ 63M with HCV cirrhosis is hospitalized due to worsened fluid retention, with ascites and lower extremity edema ■ He denies fever or frank abdominal pain, though is uncomfortable from abdominal distension. ■ He finds it difficult to walk as a result of severe leg edema Case 1 ■ VS: T37 HR 65 BP 110/70 RR 20 SpO2 98% ■ Gen: chronically ill ■ CV: 3+ BLE edema, anasarca ■ Resp: normal other than decreased BS at bases ■ GI: distended abdomen with dullness to percussion, nontender ■ Labs: WBC 4, hct 32, plt 60, INR 1.9, Na 122, Cr 2.5, total bili 6, albumin 2.8 Case 1 ■ What is your strategy for management of this patient’s volume overload? ■ How would you handle his hyponatremia? Approach to hyponatremia in cirrhosis Attar, CLD, 2019. IV albumin leads to resolution of hyponatremia Bajaj, AJG, 2018. IV albumin and ascites Treatments Study Outcomes sample ANSWER Albumin 40g n = 431 IRR for death 0.61 BIW x 2 weeks On favoring albumin then 40g/wk + diuretics, SMT vs ascites SMT alone MACHT Albumin 40g q n = 196 No difference in survival 15d + midodrine (only 173 or liver complications vs placebo analyzed) More LT in albumin Listed for group (68% vs 55%; LT, p=0.08) ascites Outpatient IV albumin use may improve survival and hospitalization Mortality Hospitalization Di Pascoli, Liver Int, 2018. -

Gastrointestinal Bleeding Gary A
Article gastroenterology Gastrointestinal Bleeding Gary A. Neidich, MD* Educational Gaps Sarah R. Cole, MD* 1. Pediatricians should be familiar with diseases that may present with gastrointestinal bleeding in patients at varying ages. Author Disclosure 2. Pediatricians should be aware of newer technologies for the identification and therapy Drs Neidich and Cole of gastrointestinal bleeding sources. have disclosed no 3. Pediatricians should be familiar with polyps that have and do not have an increased financial relationships risk of malignant transformation. relevant to this article. 4. Pediatricians should be familiar with medications used in the treatment of children This commentary does with gastrointestinal bleeding. not contain a discussion of an Objectives After completing this article, readers should be able to: unapproved/ investigative use of 1. Formulate a diagnostic and management plan for children with gastrointestinal a commercial product/ bleeding. device. 2. Describe newer techniques and their limitations for the identification of bleeding, including small intestinal capsule endoscopy and small intestinal enteroscopy. 3. Differentiate common and less common causes of gastrointestinal bleeding in children of varying ages. 4. Identify types of polyps that may present in childhood and which of these have malignant potential. Introduction An 11-year-old boy is seen in the emergency department after fainting at home. He has a 2-day history of headache and dizziness. Epigastric pain has been present during the past 2 days. His pulse is 150 beats per minute, and his blood pressure is 90/50 mm Hg. An in- travenous bolus of normal saline is administered; his hemoglobin level is 8.1 g/dl (81 g/L).