(NE MEDITERRANEAN) (Two Volumes) SEID
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Evaluating the Risk to Ramsar Sites from Climate Change Induced Sea
STRP Scientific and Technical Briefing Note Review Panel Number 5, December 2012 Ramsar Convention on Wetlands Purpose of this BN Evaluating the risk to Ramsar Sites from To provide a preliminary as- climate change induced sea level rise sessment of coastal Ramsar Sites that are at risk to inunda- his Briefing Note and the accompanying web map service and data tion as a consequence of sea Tsets, developed by the Center for International Earth Science Infor- level rise, in order to provide mation Network (CIESIN) of Columbia University, provide a preliminary site managers with information that may assist them in assess- assessment of the risk to coastal wetlands designated as Wetlands of ing adaptation strategies. International Importance (Ramsar Sites) under the Ramsar Convention on Wetlands from rising sea levels due to climate change. Two scenarios are evaluated, 0-1 meter sea level rise (SLR), which is close to what the Background information Intergovernmental Panel on Climate Change (IPCC) predicts for this cen- tury, and 0-2 meter SLR, which is an upper bound for SLR in this century CIESIN, through its NASA- if land-based ice sheets respond faster than expected to temperature supported Socioeconomic changes (AMAP 2011, Pfeffer et al. 2008). It has to be recognized that Data and Applications Center (SEDAC), conducted a glo- sea level rise will not be consistent globally, but is affected by coastal bal analysis of Ramsar Sites bathymetry and local topography and tides, while the extent of areas and developed this report in periodically submerged will also be affected by storm surges (Strauss et response to a request by the al. -
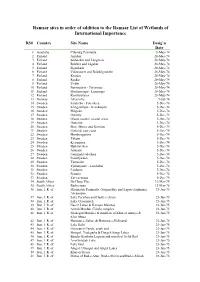
Ramsar Sites in Order of Addition to the Ramsar List of Wetlands of International Importance
Ramsar sites in order of addition to the Ramsar List of Wetlands of International Importance RS# Country Site Name Desig’n Date 1 Australia Cobourg Peninsula 8-May-74 2 Finland Aspskär 28-May-74 3 Finland Söderskär and Långören 28-May-74 4 Finland Björkör and Lågskär 28-May-74 5 Finland Signilskär 28-May-74 6 Finland Valassaaret and Björkögrunden 28-May-74 7 Finland Krunnit 28-May-74 8 Finland Ruskis 28-May-74 9 Finland Viikki 28-May-74 10 Finland Suomujärvi - Patvinsuo 28-May-74 11 Finland Martimoaapa - Lumiaapa 28-May-74 12 Finland Koitilaiskaira 28-May-74 13 Norway Åkersvika 9-Jul-74 14 Sweden Falsterbo - Foteviken 5-Dec-74 15 Sweden Klingavälsån - Krankesjön 5-Dec-74 16 Sweden Helgeån 5-Dec-74 17 Sweden Ottenby 5-Dec-74 18 Sweden Öland, eastern coastal areas 5-Dec-74 19 Sweden Getterön 5-Dec-74 20 Sweden Store Mosse and Kävsjön 5-Dec-74 21 Sweden Gotland, east coast 5-Dec-74 22 Sweden Hornborgasjön 5-Dec-74 23 Sweden Tåkern 5-Dec-74 24 Sweden Kvismaren 5-Dec-74 25 Sweden Hjälstaviken 5-Dec-74 26 Sweden Ånnsjön 5-Dec-74 27 Sweden Gammelstadsviken 5-Dec-74 28 Sweden Persöfjärden 5-Dec-74 29 Sweden Tärnasjön 5-Dec-74 30 Sweden Tjålmejaure - Laisdalen 5-Dec-74 31 Sweden Laidaure 5-Dec-74 32 Sweden Sjaunja 5-Dec-74 33 Sweden Tavvavuoma 5-Dec-74 34 South Africa De Hoop Vlei 12-Mar-75 35 South Africa Barberspan 12-Mar-75 36 Iran, I. R. -

Las Primacías Eclesiásticas En Hispania Durante El Siglo IV
POLIS. Revista de ideas y formas políticas de la Antigüedad Clásica 10, 1998, pp. 269-285. LAS PRIMACÍAS ECLESIÁSTICAS EN HZSPA2VIA DURANTE EL SIGLO IV* Josep 'VUella Masana Universidad de Barcelona La historia del cristianismo antiguo está orgánicamente vinciolada a la del Imperio romano, en el que nace, se desarrolla y se convierte en institución. Entre ambos se ftagua una relación que, con el paso de los años, se hace cada vez más unívoca, recíproca. Con el fracaso del régimen instaurado por Diocleciano y el apoyo al cristianismo por parte de Constantino 1, tiene lugar el paso del Imperio pagano al Imperio cristiano. Lo más novedoso no es la finalización de las persecuciones —que ya hacía años que no se producían en muchas zonas— sino el hecho de que se legisle a fevor de los cristianos — sobre todo de los eclesiásticos— y que prácticas cristianas de laiga tradición alcancen categoría de ley^ De modo parecido sucedió con * Este estudio se ha realizado en el marco de los proyectos y grupos de investigación PB97- 0891 y 1997SGR 357. El presente texto corresponde a nuestra qxjrtación al coloquio internacional dedicado a "Paciano y la Hispania cristiana del siglo IV", celebrado en Barcelona del 8 al 9 de marzo de 1996 y en Lyón del 28 al 30 de octubre del mismo año. 269 Josep Vilella Masana disposiciones de orden organizativo que el episcopado establece al convertirse en un estamento público y privilegiado. Sería éste el caso de algunos aspectos que, en el 325, re¿amenta el concilio de Nicea^. En Nicea se establece la agrupación de los obispos de una misma provincia bajo la presidencia del obispo de la capital provincial, denominado metropolitano. -

1St International Eurasian Ornithology Congress
1st International Eurasian Ornithology Congress Erdoğan, A., Turan, L., Albayrak, T. (Ed.) 1ST INTERNATIONAL EURASIAN ORNITHOLOGY CONGRESS Antalya, Turkey 8-11 April 2004 Jointly organized by Akdeniz University - Antalya and Hacettepe University - Ankara i 1st International Eurasian Ornithology Congress Ali Erdoğan, Levent Turan, Tamer Albayrak (Editorial Board) 1ST INTERNATIONAL EURASIAN ORNITHOLOGY CONGRESS Antalya Turkey 8-11 April 2004 ISBN: 975-98424-0-8 Print: Sadri Grafik 2004 Antalya ii 1st International Eurasian Ornithology Congress HONORARY PRESIDENTS (ALPHABETICALLY ORDERED) Prof. Dr. Tunçalp ÖZGEN Rector of Hacettepe University, Ankara Prof.Dr.Yaşar UÇAR Rector of Akdeniz University, Antalya CONGRESS CHAIRMAN Prof.Dr. İlhami KİZİROĞLU Hacettepe University EXECUTİVE COMMİTTEE Prof. Dr. Ali ERDOĞAN (Chairman) Prof. Dr. İlhami KİZİROĞLU Assoc. Prof. Dr. Levent TURAN (Vice Chairman) Cengiz GÖKOĞLU (Mayor of Bogazkent ) SCIENTIFIC CONGRESS SECRETARY Tamer ALBAYRAK (Akdeniz University, Antalya) iii 1st International Eurasian Ornithology Congress SCIENTIFIC COMMITTEE Özdemir ADIZEL, (Yüzüncüyıl U. Van, Turkey ) Zafer AYAŞ, (Hacettepe U. Ankara, Turkey) Yusuf AYVAZ, (S. Demirel U. Isparta,Turkey) Walter BÄUMLER, (TU, Münich, Germany ) Franz BAIRLEIN, (Journal f.Ornithologie, Germany) Stuart BEARHOP, (University of Glasgow, UK) Einhard BEZZEL, (Falke, Germany) Mahmut BILGINER, (Ondokuz Mayıs U. Samsun, Turkey) Dan CHAMBERLAIN, (University of Stirling, UK) Ali ERDOĞAN, (Akdeniz U. Antalya, Turkey) Michael EXO, (Institut fuer Vogelforschung, -

Farming in Mediterranean France and Rural Settlement in The
Farming in mediterranean France and rural settlement in the Late Roman and early Medieval periods : the contribution from archaeology and environmental sciences in the last twenty years Aline Durand, Philippe Leveau To cite this version: Aline Durand, Philippe Leveau. Farming in mediterranean France and rural settlement in the Late Roman and early Medieval periods : the contribution from archaeology and environmental sciences in the last twenty years. Miquel BARCELÓ; François SIGAUT. The Making of Feudal Agricultures ?, BRILL, pp.177-253, 2004, The transformation of the Roman World, 90-04-11722-9. halshs-01052529 HAL Id: halshs-01052529 https://halshs.archives-ouvertes.fr/halshs-01052529 Submitted on 28 Jul 2014 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Article published in BARCELÓ M. et SIGAUT F., (eds.), The Making of Feudal Agricultures ?, Boston-Leiden, Brill editor, coll. The transformation of the Roman World, vol. 14, 2004, p. 177-253. BARCELÓ M. et SIGAUT F., (eds.), The Making of Feudal Agricultures ?, Boston-Leiden, Brill editor, coll. The transformation of the Roman World, vol. 14, 2004, p. 177-253. Aline DURAND ([email protected]) Philippe LEVEAU ([email protected]) FARMING IN MEDITERRANEAN FRANCE AND RURAL SETTLEMENT IN THE LATE ROMAN AND EARLY MEDIEVAL PERIODS: THE CONTRIBUTION FROM ARCHAEOLOGY AND ENVIRONMENTAL SCIENCES IN THE LAST TWENTY YEARS Introduction From the end of the 19th C, French historiography has studied the period spanning the 5th - 10th C essentially along political and institutional lines. -

Sustainable Land Management
Sustainable Land Management . Selim Kapur Á Hari Eswaran Á W. E. H. Blum Editors Sustainable Land Management Learning from the Past for the Future Editors Dr. Selim Kapur Dr. Hari Eswaran Department of Soil Science & United States Department of Agriculture Archaeometry Natural Resources Conservation Service University of C¸ukurova PO Box 2890 01330 Adana, Turkey Washington, DC, USA [email protected] [email protected] Dr. Winfried E. H. Blum Institute of Soil Research Department of Forest and Soil Sciences University of Natural Resources and Applied Life Sciences (BOKU) Vienna, Austria [email protected]; [email protected] ISBN 978-3-642-14781-4 e-ISBN 978-3-642-14782-1 DOI 10.1007/978-3-642-14782-1 Springer Heidelberg Dordrecht London New York Library of Congress Control Number: 2010938610 # Springer-Verlag Berlin Heidelberg 2011 This work is subject to copyright. All rights are reserved, whether the whole or part of the material is concerned, specifically the rights of translation, reprinting, reuse of illustrations, recitation, broadcasting, reproduction on microfilm or in any other way, and storage in data banks. Duplication of this publication or parts thereof is permitted only under the provisions of the German Copyright Law of September 9, 1965, in its current version, and permission for use must always be obtained from Springer. Violations are liable to prosecution under the German Copyright Law. The use of general descriptive names, registered names, trademarks, etc. in this publication does not imply, even in the absence of a specific statement, that such names are exempt from the relevant protective laws and regulations and therefore free for general use. -

Calendar of Roman Events
Introduction Steve Worboys and I began this calendar in 1980 or 1981 when we discovered that the exact dates of many events survive from Roman antiquity, the most famous being the ides of March murder of Caesar. Flipping through a few books on Roman history revealed a handful of dates, and we believed that to fill every day of the year would certainly be impossible. From 1981 until 1989 I kept the calendar, adding dates as I ran across them. In 1989 I typed the list into the computer and we began again to plunder books and journals for dates, this time recording sources. Since then I have worked and reworked the Calendar, revising old entries and adding many, many more. The Roman Calendar The calendar was reformed twice, once by Caesar in 46 BC and later by Augustus in 8 BC. Each of these reforms is described in A. K. Michels’ book The Calendar of the Roman Republic. In an ordinary pre-Julian year, the number of days in each month was as follows: 29 January 31 May 29 September 28 February 29 June 31 October 31 March 31 Quintilis (July) 29 November 29 April 29 Sextilis (August) 29 December. The Romans did not number the days of the months consecutively. They reckoned backwards from three fixed points: The kalends, the nones, and the ides. The kalends is the first day of the month. For months with 31 days the nones fall on the 7th and the ides the 15th. For other months the nones fall on the 5th and the ides on the 13th. -

Remote Sensing and Geosciences for Archaeology
Books Remote Sensing and Geosciences for Archaeology Edited by Deodato Tapete Printed Edition of the Special Issue Published in Geosciences www.mdpi.com/journal/geosciences MDPI Remote Sensing and Geosciences for Archaeology Books Special Issue Editor Deodato Tapete MDPI • Basel • Beijing • Wuhan • Barcelona • Belgrade MDPI Special Issue Editor Deodato Tapete Italian Space Agency (ASI) Italy Editorial Office MDPI AG St. Alban-Anlage 66 Basel, Switzerland This edition is a reprint of the Special Issue published online in the open access journal Geosciences (ISSN 2076-3263) from 2017–2018 (available at: http://www.mdpi.com/journal/geosciences/special_issues/archaeology). For citation purposes, cite each article independently as indicated on the article page online and as indicated below: Books Lastname, F.M.; Lastname, F.M. Article title. Journal Name Year, Article number, page range. First Edition 2018 ISBN 978-3-03842-763-6 (Pbk) ISBN 978-3-03842-764-3 (PDF) Articles in this volume are Open Access and distributed under the Creative Commons Attribution license (CC BY), which allows users to download, copy and build upon published articles even for commercial purposes, as long as the author and publisher are properly credited, which ensures maximum dissemination and a wider impact of our publications. The book taken as a whole is © 2018 MDPI, Basel, Switzerland, distributed under the terms and conditions of the Creative Commons license CC BY-NC-ND (http://creativecommons.org/licenses/by-nc-nd/4.0/). MDPI Table of Contents About the Special Issue Editor ..................................................................................................................... vii Preface to “Remote Sensing and Geosciences for Archaeology” ........................................................... ix Deodato Tapete Remote Sensing and Geosciences for Archaeology Reprinted from: Geosciences 2018, 8(2), 41; doi: 10.3390/geosciences8020041 ...................................... -

Download From
Information Sheet on Ramsar Wetlands (RIS) – 2006-2008 version Available for download from http://www.ramsar.org/ris/key_ris_index.htm. Categories approved by Recommendation 4.7 (1990), as amended by Resolution VIII.13 of the 8th Conference of the Contracting Parties (2002) and Resolutions IX.1 Annex B, IX.6, IX.21 and IX. 22 of the 9 th Conference of the Contracting Parties (2005). Notes for compilers: 1. The RIS should be completed in accordance with the attached Explanatory Notes and Guidelines for completing the Information Sheet on Ramsar Wetlands. Compilers are strongly advised to read this guidance before filling in the RIS. 2. Furtherinformation and guidance in support of Ramsarsite designations are provided in the Strategic Framework and guidelines for the future development of the List of Wetlands of International Importance (RamsarWise Use Handbook 7, 2 nd edition, as amended by COP9 Resolution IX.1 Annex B). A 3 rd edition of the Handbook, incorporating these amendments, is in preparation and will be available in 2006. 3. Once completed, the RIS (and accompanying map(s)) should be submitted to the Ramsar Secretariat. Compilers should provide an electronic (MS Word) copy of the RIS and, where possible, digital copies of all maps. 1. Name and address of the compiler of this form: FOR OFFICE USE ONLY . DD MM YY Mehmet GÖLGE Environment & Forest Expert Address: (Ministry of Environment & Forestry) Çevre ve Orman Bakanlığı Designation date Site Reference Number Söğütözü Caddesi No: 14/E Beştepe-ANKARA/TURKEY Phone: +90.312.2075906 Fax: +90.312.2075959 E-mail: [email protected] ; [email protected] 2. -

Organochlorine Pesticides and Polychlorinated
Research Article Mediterranean Marine Science Indexed in WoS (Web of Science, ISI Thomson) and SCOPUS The journal is available online at http://www.medit-mar-sc.net DOI: http://dx.doi.org/10.12681/mms.15516 Organochlorine pesticides and polychlorinated biphenyls in Blue crabs Callinectes sapidus (Rathbun, 1896) from Akyatan lagoon in the Eastern Mediterranean region of Turkey BIGE GULMEN KILERCIOGLU1, IBRAHIM CENGIZLER1, NEBILE DAGLIOGLU2 and SERDAR KILERCIOGLU3 1 Faculty of Fisheries, University of Cukurova, 01130, Balcali- Saricam Adana, Turkey 2 Faculty of Medicine, University of Cukurova, 01130, Balcali- Saricam Adana, Turkey 3 Biotechnology Research Center University of Cukurova, 01130, Balcali- Saricam Adana, Turkey Corresponding author: [email protected] Handling Editor: Kostas Kapiris Received: 20 December 2017; Accepted: 12 May 2018; Published on line: 24 July 2018 Abstract The aim of this study is to determine the levels of organochlorine-based pesticides and polychlorinated biphenyls in the edible muscle tissue of Blue crabs Callinectes sapidus (Rathbun, 1896) that are harvested from Akyatan Lagoon in the Eastern Medi- terranean Region of Turkey. The crabs were harvested in October 2010, January 2011 and March 2011. A total of fifty crabs was studied. A quantitative determination of residue levels was carried out through a Gas Chromatography-Electron Capture Detector (GC-ECD), and compared with acceptable contaminant levels. PCBs were the greatest source of contamination. The predominant compounds were α-HCH, o,p’-DDE, PCB 28 (2,2’,4,4’-PCB) and PCB 52 (2,2’,5,5’-PCB), with mean concentrations of 22.39, 59.45, 347.31 and 362.86 ng/g wet weights, respectively. -

I. Uluslararası Akdeniz Sempozyumu 1. International Mediterranean Symposium
I. Uluslararası Akdeniz Sempozyumu 1. International Mediterranean Symposium BİLDİRİ TAM METİNLERİ KİTABI SYMPOSIUM FULL TEXT BOOK EDİTÖR Prof. Dr. Durmuş Ali ARSLAN Editör Yardımcıları Gülten ARSLAN Halil ÇAKIR MERSİN I. Uluslararası Akdeniz Sempozyumu 1. International Mediterranean Symposium BİLDİRİ TAM METİNLERİ KİTABI SYMPOSIUM FULL TEXT BOOK Editör: Prof. Dr. D. Ali ARSLAN Editör Yardımcısı: Gülten ARSLAN Halil ÇAKIR Kapak Tasarımı: Prof. Dr. D. Ali ARSLAN Mizanpaj-Ofset Hazırlık: Prof. Dr. D. Ali ARSLAN © Mer Ak Yayınları 2018 – Mersin ISBN: 978-605-89406-5-9 Mer-Ak Mersin Akademi Yayınları Adres: Çiftlikköy Mahallesi, 34. Cadde, Nisa 1 Evleri, No: 35, 6/12, Yenişehir/MERSİN Tel: 0532 270 81 45 / 0553 666 06 06 Not: Bölümlerin her türlü idari, akademik ve hukuki sorumluluğu yazarlarına aittir. 1 Önsöz Çok Değerli Bilim İnsanları ve Kıymetli Araştırmacılar, Her yıl periyodik olarak düzenlenmesi planlanan Uluslararası Akdeniz Sempozyumu’nun ilki, 1-3 Kasım 2018 tarihleri arasında Mersin’de, Mersin Üniversitesi ve Mersin Akademi Danışmanlık iş birliği ile gerçekleştirildi. Özelde Akdeniz Bölgesi illeri (Adana, Antalya, Burdur, Hatay, Isparta, Kahramanmaraş, Mersin, Osmaniye dâhil) ile bu illerimizin ilçeleri ve her türlü yerleşim birimlerini ele alan, genelde ise aşağıdaki alanlara giren her türlü bilimsel araştırma ve akademik çalışmaya sempozyum kapsamında yer verildi. Akdeniz Bölgesi, sahip olduğu zengin tarihsel miras, eşsiz doğa güzellikleri ve zengin kültürel birikimiyle yalnızca Türkiye’nin değil, dünyanın en önemli yaşam alanlarındandır. Mersin Akademi ve Mersin Üniversitesi işbirliği ile bu eşsiz coğrafyaya yönelik olarak düzenlenen bu uluslararası sempozyumda, geçmişten geleceğe Akdeniz Bölgesi’ne, Çukurova’ya, Doğu Akdeniz Havzası’na ilgi duyan, bu güzide coğrafyaya gönül bağı olan siz akademisyen ve araştırmacıları aynı çatı altında, akademik bir ortamda buluşturmaktan onur ve mutluluk duyduk. -

(Gambusia Holbrooki Girard, 1859) in 23 River Basins of Turkey
Turkish Journal of Zoology Turk J Zool (2020) 44: 324-334 http://journals.tubitak.gov.tr/zoology/ © TÜBİTAK Research Article doi:10.3906/zoo-2002-37 Length–weight relationships of invasive mosquitofish (Gambusia holbrooki Girard, 1859) in 23 river basins of Turkey Irmak KURTUL*, Hasan Musa SARI Marine and Inland Waters Sciences and Technology Department, Faculty of Fisheries, Ege University, İzmir, Turkey Received: 21.02.2020 Accepted/Published Online: 16.06.2020 Final Version: 13.07.2020 Abstract: Gambusia holbrooki Girard, 1859 has established very strong populations in freshwater resources in Turkey after being introduced into Amik Lake (Hatay/Antakya) at the beginning of the 1920s. In this study, it was aimed to determine the female:male ratios and length-weight relationships of G. holbrooki populations in Turkey, which are considered as a threat, especially for endemic species. G. holbrooki specimen were sampled at total of 66 locations, including 39 lentic and 27 lotic locations, between 2016 and 2017. The female:male ratio was 1:0.39 for all of the investigated localities. Length–weight relationships were investigated at 44 sampling locations for the females and 34 sampling locations for the males. The sampled specimens ranged between 12.7 and 57.2 mm in total length, and 0.03 and 2.88 g in total weight. The length-weight relationship of the female and male G. holbrooki specimens indicated mostly isometric growth, whereas the b values varied between 1.755 and 4.009, and 2.154 and 3.665 for the females and males, respectively, in terms of the sampling locations.