Atep Nurdjaman™, RAN™ Journal Full Paper August 03, 2007
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

1 Abietic Acid R Abrasive Silica for Polishing DR Acenaphthene M (LC
1 abietic acid R abrasive silica for polishing DR acenaphthene M (LC) acenaphthene quinone R acenaphthylene R acetal (see 1,1-diethoxyethane) acetaldehyde M (FC) acetaldehyde-d (CH3CDO) R acetaldehyde dimethyl acetal CH acetaldoxime R acetamide M (LC) acetamidinium chloride R acetamidoacrylic acid 2- NB acetamidobenzaldehyde p- R acetamidobenzenesulfonyl chloride 4- R acetamidodeoxythioglucopyranose triacetate 2- -2- -1- -β-D- 3,4,6- AB acetamidomethylthiazole 2- -4- PB acetanilide M (LC) acetazolamide R acetdimethylamide see dimethylacetamide, N,N- acethydrazide R acetic acid M (solv) acetic anhydride M (FC) acetmethylamide see methylacetamide, N- acetoacetamide R acetoacetanilide R acetoacetic acid, lithium salt R acetobromoglucose -α-D- NB acetohydroxamic acid R acetoin R acetol (hydroxyacetone) R acetonaphthalide (α)R acetone M (solv) acetone ,A.R. M (solv) acetone-d6 RM acetone cyanohydrin R acetonedicarboxylic acid ,dimethyl ester R acetonedicarboxylic acid -1,3- R acetone dimethyl acetal see dimethoxypropane 2,2- acetonitrile M (solv) acetonitrile-d3 RM acetonylacetone see hexanedione 2,5- acetonylbenzylhydroxycoumarin (3-(α- -4- R acetophenone M (LC) acetophenone oxime R acetophenone trimethylsilyl enol ether see phenyltrimethylsilyl... acetoxyacetone (oxopropyl acetate 2-) R acetoxybenzoic acid 4- DS acetoxynaphthoic acid 6- -2- R 2 acetylacetaldehyde dimethylacetal R acetylacetone (pentanedione -2,4-) M (C) acetylbenzonitrile p- R acetylbiphenyl 4- see phenylacetophenone, p- acetyl bromide M (FC) acetylbromothiophene 2- -5- -

In Presenting the Dissertation As a Partial Fulfillment of The
In presenting the dissertation as a partial fulfillment of the requirements for an advanced degree from the Georgia Institute of Technology, I agree that the Library of the Institute shall make it available for inspection and circulation in accordance with its regulations governing materials of this type. I agree that permission to copy from, or to publish from, this dissertation may be granted by the professor under whose direction it was written, or, in his absence, by the Dean of the Graduate Division when such copying or publication is solely for scholarly purposes and does not involve potential financial gain. It is under stood that any copying from, or publication of, this dis sertation which involves potential financial gain will not be allowed without written permission. 7/25/68 SYNTHESES AND REARRANGEMENTS OF ORGANOALKALI COMPOUNDS A THESIS Presented to the Faculty of the Graduate Division by Yao-Ming Cheng In Partial Fulfillment of the Requirements for the Degree Doctor of Philosophy in the School of Chemistry Georgia Institute of Technology September, 1970 SYNTHESES AND REARRANGEMENTS OF ORGANOALKALI COMPOUNDS < o u >- ui Q Z >- CQ < Z o < z ui Approved: z I- >- co a z o ca Date applied by Chairman ACKNOWLEDGMENTS The author wishes to express his sincere appreciation to Dr. Erling Grovenstein. Jr. for his suggestion of this problem and for his advice and assistance during the course of this work. The author wishes to thank Dr. Drury S. Caine and Dr. Charles L. Liotta for serving as members of the reading committee. The Graduate Assistantships provided by the Department of Chemistry, which were supported by the National Science Foundation and the National Aeronautics and Space Administration and were held by the author during the course of this research, are greatly appreciated. -

Biochemical Investigations in the Rare Disease Alkaptonuria: Studies on the Metabolome and the Nature of Ochronotic Pigment
Biochemical Investigations in the Rare Disease Alkaptonuria: Studies on the Metabolome and the Nature of Ochronotic Pigment Thesis submitted in accordance with the requirements of the University of Liverpool for the degree of Doctor of Philosophy by Brendan Paul Norman September 2019 ACKNOWLEDGEMENTS It is hard to describe the journey this PhD has taken me on without reverting to well-worn clichés. There has been plenty of challenges along the way, but ultimately I can look back on the past four years with a great sense of pride, both in the work presented here and the skills I have developed. Equally important though are the relationships I have established. I have lots of people to thank for playing a part in this thesis. First, I would like to thank my supervisors, Jim Gallagher, Lakshminarayan Ranganath and Norman Roberts for giving me this fantastic opportunity. Your dedication to research into alkaptonuria (AKU) is inspiring and our discussions together have always been thoughtful and often offered fresh perspective on my work. It has been a pleasure to work under your supervision and your ongoing support and encouragement continues to drive me on. It has truly been a pleasure to be part of the AKU research group. Andrew Davison deserves a special mention - much of the highs and lows of our PhD projects have been experienced together. Learning LC-QTOF-MS was exciting (and continues to be) but equally daunting at the start of our projects (admittedly more so for me as a Psychology graduate turned mass spectrometrist!). I am very proud of what we have achieved together, largely starting from scratch on the instrument, and we are continuing to learn all the time. -

Working with Hazardous Chemicals
A Publication of Reliable Methods for the Preparation of Organic Compounds Working with Hazardous Chemicals The procedures in Organic Syntheses are intended for use only by persons with proper training in experimental organic chemistry. All hazardous materials should be handled using the standard procedures for work with chemicals described in references such as "Prudent Practices in the Laboratory" (The National Academies Press, Washington, D.C., 2011; the full text can be accessed free of charge at http://www.nap.edu/catalog.php?record_id=12654). All chemical waste should be disposed of in accordance with local regulations. For general guidelines for the management of chemical waste, see Chapter 8 of Prudent Practices. In some articles in Organic Syntheses, chemical-specific hazards are highlighted in red “Caution Notes” within a procedure. It is important to recognize that the absence of a caution note does not imply that no significant hazards are associated with the chemicals involved in that procedure. Prior to performing a reaction, a thorough risk assessment should be carried out that includes a review of the potential hazards associated with each chemical and experimental operation on the scale that is planned for the procedure. Guidelines for carrying out a risk assessment and for analyzing the hazards associated with chemicals can be found in Chapter 4 of Prudent Practices. The procedures described in Organic Syntheses are provided as published and are conducted at one's own risk. Organic Syntheses, Inc., its Editors, and its Board of Directors do not warrant or guarantee the safety of individuals using these procedures and hereby disclaim any liability for any injuries or damages claimed to have resulted from or related in any way to the procedures herein. -
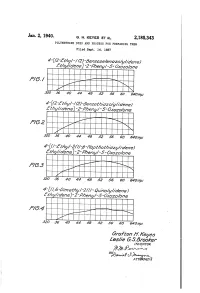
Ârofïon H. Keyes ¿Ès//È 6 5
Jan.. 2„ 1940. A G. H. KEYES E1- AL 2,185,343 POLYMETHINE DYÉS AND PROCESS FOR PREPARING THEM Filed Sept. 14, 1937 .320 36 40 44 48 .52 .5 6 60 64007,!! 320 .i6 40 44 4a 52 56 60 E40/fp `320 36 40 44’ v48 .52 56 60 640030 ârofïon H. Keyes ¿ès//è 6 5. ßmokef‘ l N VEN TOR Patented Jan. 2, 1940 2,185,343 UNITED STATES PATENT oFFIcE 2,185,343 POLYMIETHINE D‘YES ANDl PROCESS FOR l PREPARING THEM Grafton H. Keyes and Leslie G. S. Brooker, a. Rochester, N. Y., assignors to Eastman Kodak Company, Rochester, N. Y., a corporation of New Jersey Application ‘September 14, 1937, Serial No. 163,782 In Great Britain September 15, 1936 10 Claims. (Cl. 260-240) This invention relates to new dyes and to Our new dyes are probably resonance hybrids photographic emulsions sensitized therewith. between two forms which can be illustrated for We have found that new dyes can be prepared the dyes of Formula IIa.` as follows: by reacting a monoacylated aminoacetic acid with a formylmethylene compound of one of the following formulas: wherein D represents the non-metallic atoms necessary to complete a heterocyclic nucleus, such 15 as a pyridine or a quinoline nucleus for example, Z represents the non-metallic atoms necessary to complete a heterocyclic nucleus, such as a The dyes of Formula IIb can similarly be ex pressed as resonance hybrids between two forms. 20 live-membered or siX-membered heterocyclic nucleus for example, and R represents an ali In the instant application, we shall formulate our phatic radical, i. -

The Condensation of Aromatic Aldehydes with Glycine and Acetylglycine
THE CONDENSATION OF AROMATIC ALDEHYDES WITH GLYCINE AND ACETYLGLYCINE. BY H. D. DAKIN. (From Scarborough-on-Hudson, New York.) (Received for publication, March 29, 1929.) The condensation of aromatic aldehydes with acyl derivatives of glycine has, in its various modifications, furnished the most useful method available for the synthesis of compounds which may in turn be converted into aromatic amino and ketonic acids such as tyrosine, tryptophane, and thyroxine. The reaction was first studied by Plochl (1) who condensed hippuric acid with Downloaded from benzaldehyde and salicylic aldehyde. The correct interpretation of this reaction and the demonstration of its ability for amino acid synthesis was furnished later by Erlenmeyer (2). Instead of hippuric acid, later investigators (3) have used other derivatives of glycine such as hydantoin and glycine anhydride. In connec- www.jbc.org tion with an investigation of aldehyde derivatives of amino acids, peptides, and proteins it became of interest to examine the be- havior of glycine itself when condensed with benzaldehyde and acetic anhydride. The reaction was first studied by Plochl (1) by on May 22, 2009 who stated that: “Es konnten jedoch daraus auf keine Weise zur Untersuchung einladende Korper sondern nur Schmieren er- halten werden.” In fact it was this failure to effect the condensa- tion of glycine that led Plochl to use hippuric acid in its place. Later on Erlenmeyer and Friistiick (4) succeeded in isolating the azlactone of a-acetaminocinnamic acid and subsequently Berg- mann and Stern (5), by varying the conditions, obtained 44 to 50 per cent yields and showed how valuable this and the related derivative from p-hydroxybenzaldehyde were for the synthesis of amino acids and peptides. -

Www .Alfa.Com
Bio 2013-14 Alfa Aesar North America Alfa Aesar Korea Uni-Onward (International Sales Headquarters) 101-3701, Lotte Castle President 3F-2 93 Wenhau 1st Rd, Sec 1, 26 Parkridge Road O-Dong Linkou Shiang 244, Taipei County Ward Hill, MA 01835 USA 467, Gongduk-Dong, Mapo-Gu Taiwan Tel: 1-800-343-0660 or 1-978-521-6300 Seoul, 121-805, Korea Tel: 886-2-2600-0611 Fax: 1-978-521-6350 Tel: +82-2-3140-6000 Fax: 886-2-2600-0654 Email: [email protected] Fax: +82-2-3140-6002 Email: [email protected] Email: [email protected] Alfa Aesar United Kingdom Echo Chemical Co. Ltd Shore Road Alfa Aesar India 16, Gongyeh Rd, Lu-Chu Li Port of Heysham Industrial Park (Johnson Matthey Chemicals India Toufen, 351, Miaoli Heysham LA3 2XY Pvt. Ltd.) Taiwan England Kandlakoya Village Tel: 866-37-629988 Bio Chemicals for Life Tel: 0800-801812 or +44 (0)1524 850506 Medchal Mandal Email: [email protected] www.alfa.com Fax: +44 (0)1524 850608 R R District Email: [email protected] Hyderabad - 501401 Andhra Pradesh, India Including: Alfa Aesar Germany Tel: +91 40 6730 1234 Postbox 11 07 65 Fax: +91 40 6730 1230 Amino Acids and Derivatives 76057 Karlsruhe Email: [email protected] Buffers Germany Tel: 800 4566 4566 or Distributed By: Click Chemistry Reagents +49 (0)721 84007 280 Electrophoresis Reagents Fax: +49 (0)721 84007 300 Hydrus Chemical Inc. Email: [email protected] Uchikanda 3-Chome, Chiyoda-Ku Signal Transduction Reagents Tokyo 101-0047 Western Blot and ELISA Reagents Alfa Aesar France Japan 2 allée d’Oslo Tel: 03(3258)5031 ...and much more 67300 Schiltigheim Fax: 03(3258)6535 France Email: [email protected] Tel: 0800 03 51 47 or +33 (0)3 8862 2690 Fax: 0800 10 20 67 or OOO “REAKOR” +33 (0)3 8862 6864 Nagorny Proezd, 7 Email: [email protected] 117 105 Moscow Russia Alfa Aesar China Tel: +7 495 640 3427 Room 1509 Fax: +7 495 640 3427 ext 6 CBD International Building Email: [email protected] No. -
S TU D IE SOF Pt METALCOMP LE XESW IT H LIG
1. STUDIES OF Pt METAL COMPLEXES W ITH LIGANDS CONTAINING C, Si, Ge and Sn. By Victor Christou B.Sc., A.R.C.S.. A thesis submitted in partial fulfilment of the requirements for the awards of Doctor of Philosophy of the University of London and Diploma of Imperial College Inorganic Chemistry Laboratories, Department of Chemistry, Imperial College of Science,Technology and Medicine, London SW7 2AY. December 1990. Mum -for all the times I promised to go home but never quite made it - This is what I was doing Jbergekumene tsores iz gut tsu dertselyn . Troubles overcome are good to tell. — Yiddish Proverb. 4. A bstract. In order to evaluate the influence of N-donor ligands in neopentylplatinum(II) reactivity, a series of heteroaromatic N-donor complexes, Pt(CH2CMe 3 )2L2 , has been prepared by ligand displacement from the corresponding diene complex Pt(CH2CMe3)2(nbd). These complexes have been fully characterised by and 13C N.M.R., infrared and electronic spectroscopy. In homogeneous solution, the phenanthroline complexes are thermolytically inert to temperatures in excess of 120*C, whereupon they decompose gradually via a complex series of competing or sequential steps generating metallic platinum {inter alia). In contrast, at 90*C, Pt(CH2CMe3)2(bipy) generates an insoluble oligomeric bipyridylplatinum complex, concomitant with formation of neopentane. The nature of this product is discussed and compared with other similar bipyridylplatinum(II) species. The effect of substituent electronic effects on the rates of reductive arene elimination during metallacyclisation of neophylpladnum is also studied, using a range of mixed aryl-neophylplatinum complexes, Pt(CH2CMe2Ph)(4-C6H4R)L2 [R = H, CH3, lBu, CF3]. -

Organic Reactions V1
Organic Reactions VOLUME I EDITORIAL BOARD ROGER ADAMS, Editor-in-Chief WERNER E. BACHMANN JOHN R. JOHNSON LOUIS F. FIESER H. R. SNYDER ASSOCIATE EDITORS A. H. BLATT CHARLES R. HAUSER F. F. BLICKE MARLIN T. LEFFLER NATHAN L. DRAKE ELMORE L. MARTIN REYNOLD C. FUSON RALPH L. SHRINER LEE IRVIN SMITH NEW YORK JOHN WILEY & SONS, INC. LONDON: CHAPMAN & HALL, LIMITED 1942 COPYRIGHT, 1942 BY ROGER ADAMS All Bights Reserved This book or any part thereof must not be reproduced in any form without the written permission of the publisher. PRINTED IN THE UNITED STATES OF AMERICA PREFACE In the course of nearly every program of research in organic chemistry the investigator finds it necessary to use several of the better-known synthetic reactions. To discover the optimum conditions for the appli- cation of even the most familiar one to a compound not previously sub- jected to the reaction often requires an extensive search of the litera- ture; even then a series of experiments may be necessary. When the results of the investigation are published, the synthesis, which may have required months of work, is usually described without comment. The background of knowledge and experience gained in the literature search and experimentation is thus lost to those who subsequently have occa- sion to apply the general method. The student of preparative organic chemistry faces similar difficulties. The textbooks and laboratory man- uals furnish numerous examples of the application of various syntheses, but only rarely do they convey an accurate conception of the scope and usefulness of the processes. For many years American organic chemists have discussed these prob- lems. -

Scope and Mechanism of the Organotin Hydride Reduction of Alkyl Halides
University of New Hampshire University of New Hampshire Scholars' Repository Doctoral Dissertations Student Scholarship Fall 1964 SCOPE AND MECHANISM OF THE ORGANOTIN HYDRIDE REDUCTION OF ALKYL HALIDES LAWRENCE WILLIAM MENAPACE Follow this and additional works at: https://scholars.unh.edu/dissertation Recommended Citation MENAPACE, LAWRENCE WILLIAM, "SCOPE AND MECHANISM OF THE ORGANOTIN HYDRIDE REDUCTION OF ALKYL HALIDES" (1964). Doctoral Dissertations. 794. https://scholars.unh.edu/dissertation/794 This Dissertation is brought to you for free and open access by the Student Scholarship at University of New Hampshire Scholars' Repository. It has been accepted for inclusion in Doctoral Dissertations by an authorized administrator of University of New Hampshire Scholars' Repository. For more information, please contact [email protected]. This dissertation has been 64—7567 microfilmed exactly as received MENAPACE, Lawrence William, 1937- SCOPE AND MECHANISM OF THE ORGANOTIN HYDRIDE REDUCTION OF ALKYL HALIDES. University of New Hampshire, Ph.D., 1964 Chemistry, organic University Microfilms. Inc., Ann Arbor, Michigan SCOPE AND MECHANISM OF THE GRGANOTIN H U B IDE DEDUCTION OF AIZTL HALHES BY LAMtENGE WILLIAM MENAPACE B. S., St. PBter's College, I960 A THESIS Submitted to the University of New Hampshire In Partial Fulfillment of The Requirements for the Degree of Doctor of Philosophy Graduate School Department of Chemistry September, 1963 ACKNOWLEDGMENTS The author would like to express his deeply felt appreciation to Dr. Henry G* Kuivila, under whose direction this research was carried out, for his guidance, patience and understanding. For financial support, the author is grateful to the National Science Foundation and to M & T Chemicals, Inc* The author would also like to express his grateful ness to his wife* Her love, inspiration and help were a source of constant encouragement. -

Synthesis of Some Valine Derivatives As Potential Antibacterial Agents Frederick Nelson Minard Iowa State College
Iowa State University Capstones, Theses and Retrospective Theses and Dissertations Dissertations 1949 Synthesis of some valine derivatives as potential antibacterial agents Frederick Nelson Minard Iowa State College Follow this and additional works at: https://lib.dr.iastate.edu/rtd Part of the Biochemistry Commons, Microbiology Commons, and the Organic Chemistry Commons Recommended Citation Minard, Frederick Nelson, "Synthesis of some valine derivatives as potential antibacterial agents " (1949). Retrospective Theses and Dissertations. 13722. https://lib.dr.iastate.edu/rtd/13722 This Dissertation is brought to you for free and open access by the Iowa State University Capstones, Theses and Dissertations at Iowa State University Digital Repository. It has been accepted for inclusion in Retrospective Theses and Dissertations by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. NOTE TO USERS This reproduction is the best copy available. UMI SYNTHESIS OF SOME VALINE DERIVATIVES AS POTENTIAL ANTIBACTERIAL AGENTS by Frederick N* Minard A Dissertation Submitted to the Graduate Faculty in Partial Fulfillment of The Requirements for the Degree of DOCTOR OF PHILOSOPHY Major Subjects Oheiaist Approved! Signature was redacted for privacy. In CSiarge of/Major Work Signature was redacted for privacy. Head of Maj(^ Dei^rtment Signature was redacted for privacy. fill ti Fi(l Miir' r,! r^r . m Dean of Graduate CollegeCo] Iowa State College 19»fr9 UMI Number: DP12856 INFORMATION TO USERS The quality of this reproduction is dependent upon the quality of the copy submitted. Broken or indistinct print, colored or poor quality illustrations and photographs, print bleed-through, substandard margins, and improper alignment can adversely affect reproduction. -

Naming and Indexing of Chemical Substances for Chemical Abstractstm
Naming and Indexing of Chemical Substances for Chemical AbstractsTM 2007 Edition A publication of Chemical Abstracts Service Published by the American Chemical Society Naming and Indexing of Chemical Substances for Chemical AbstractsTM A publication of Chemical Abstracts Service Published by the American Chemical Society Copyright © 2008 American Chemical Society All Rights Reserved. Printed in the USA Inquiries concerning editorial content should be sent to: Editorial Office, Chemical Abstracts Service, 2540 Olentangy River Road, P.O. Box 3012, Columbus, Ohio 43210-0012 USA SUBSCRIPTION INFORMATION Questions about CAS products and services should be directed to: United States and Canada: CAS Customer Care Phone: 800-753-4227 (North America) 2540 Olentangy River Road 614-447-3700 (worldwide) P.O. Box 3012 Fax: 614-447-3751 Columbus, Ohio 43210-0012 USA E-mail: [email protected] Japan: JAICI (Japan Association for International Phone: 81-3-5978-3621 Chemical Information) Fax: 81-3-5978-3600 6-25-4 Honkomagome E-mail: [email protected] Bunkyo-ku, Tokyo Japan, 113-0021 Countries not named above: Contact CAS Customer Care, 2540 Olentangy River Road, P.O. Box 3012, Columbus, Ohio 43210-0012 USA; Telephone 614-447-3700; Fax 614-447-3751; E-mail [email protected]. For a list of toll-free numbers from outside North America, visit www.cas.org. 1 Naming and Indexing of Chemical Substances for Chemical Abstracts 2007 ¶ 102 NAMING AND INDEXING OF CHEMICAL SUBSTANCES 101. Foreword. Although the account which follows describes in consid- zwitterions (inner salts, sydnones). The changes for the Fourteenth (1997- erable detail the selection of substance names for Chemical Abstracts (CA) in- 2001) Collective Index period affect coordination nomenclature, stereochemi- dexes, it is not a nomenclature manual.