Spectrum of Biopsy Proven Renal Diseases in Central China
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

PATHOLOGY of the RENAL SYSTEM”, I Hope You Guys Like It
ﺑﺴﻢ اﷲ اﻟﺮﺣﻤﻦ اﻟﺮﺣﯿﻢ ھﺬه اﻟﻤﺬﻛﺮة ﻋﺒﺎرة ﻋﻦ إﻋﺎدة ﺗﻨﺴﯿﻖ وإﺿﺎﻓﺔ ﻧﻮﺗﺎت وﻣﻮاﺿﯿﻊ ﻟﻤﺬﻛﺮة زﻣﻼﺋﻨﺎ ﻣﻦ اﻟﺪﻓﻌﺔ اﻟﺴﺎﺑﻘﺔ ٤٢٧ اﻷﻋﺰاء.. ﻟﺘﺘﻮاﻓﻖ ﻣﻊ اﻟﻤﻨﮭﺞ اﻟﻤﻘﺮر ﻣﻦ اﻟﻘﺴﻢ ﺣﺮﺻﻨﺎ ﻓﯿﮭﺎ ﻋﻠﻰ إﻋﺎدة ﺻﯿﺎﻏﺔ ﻛﺜﯿﺮ ﻣﻦ اﻟﺠﻤﻞ ﻟﺘﻜﻮن ﺳﮭﻠﺔ اﻟﻔﮭﻢ وﺳﻠﺴﺔ إن ﺷﺎء اﷲ.. وﺿﻔﻨﺎ ﺑﻌﺾ اﻟﻨﻮﺗﺎت اﻟﻤﮭﻤﺔ وأﺿﻔﻨﺎ ﻣﻮاﺿﯿﻊ ﻣﻮﺟﻮدة ﺑﺎﻟـ curriculum ﺗﻌﺪﯾﻞ ٤٢٨ ﻋﻠﻰ اﻟﻤﺬﻛﺮة ﺑﻮاﺳﻄﺔ اﺧﻮاﻧﻜﻢ: ﻓﺎرس اﻟﻌﺒﺪي ﺑﻼل ﻣﺮوة ﻣﺤﻤﺪ اﻟﺼﻮﯾﺎن أﺣﻤﺪ اﻟﺴﯿﺪ ﺣﺴﻦ اﻟﻌﻨﺰي ﻧﺘﻤﻨﻰ ﻣﻨﮭﺎ اﻟﻔﺎﺋﺪة ﻗﺪر اﻟﻤﺴﺘﻄﺎع، وﻻ ﺗﻨﺴﻮﻧﺎ ﻣﻦ دﻋﻮاﺗﻜﻢ ! 2 After hours, or maybe days, of working hard, WE “THE PATHOLOGY TEAM” are proud to present “PATHOLOGY OF THE RENAL SYSTEM”, I hope you guys like it . Plz give us your prayers. Credits: 1st part = written by Assem “ THe AWesOme” KAlAnTAn revised by A.Z.K 2nd part = written by TMA revised by A.Z.K د.ﺧﺎﻟﺪ اﻟﻘﺮﻧﻲ 3rd part = written by Abo Malik revised by 4th part = written by A.Z.K revised by Assem “ THe AWesOme” KAlAnTAn 5th part = written by The Dude revised by TMA figures were provided by A.Z.K Page styling and figure embedding by: If u find any error, or u want to share any idea then plz, feel free to msg me [email protected] 3 Table of Contents Topic page THE NEPHROTIC SYNDROME 4 Minimal Change Disease 5 MEMBRANOUS GLOMERULONEPHRITIS 7 FOCAL SEGMENTAL GLOMERULOSCLEROSIS 9 MEMBRANOPROLIFERATIVE GLOMERULONEPHRITIS 11 DIABETIC NEPHROPATHY (new) 14 NEPHRITIC SYNDROME 18 Acute Post-infectious GN 19 IgA Nephropathy (Berger Disease) 20 Crescentic GN 22 Chronic GN 24 SLE Nephropathy (new) 26 Allograft rejection of the transplanted kidney (new) 27 Urinary Tract OBSTRUCTION, 28 RENAL STONES 23 HYDRONEPHROSIS -

Glomerulonephritis Management in General Practice
Renal disease • THEME Glomerulonephritis Management in general practice Nicole M Isbel MBBS, FRACP, is Consultant Nephrologist, Princess Alexandra lomerular disease remains an important cause Hospital, Brisbane, BACKGROUND Glomerulonephritis (GN) is an G and Senior Lecturer in important cause of both acute and chronic kidney of renal impairment (and is the commonest cause Medicine, University disease, however the diagnosis can be difficult of end stage kidney disease [ESKD] in Australia).1 of Queensland. nikky_ due to the variability of presenting features. Early diagnosis is essential as intervention can make [email protected] a significant impact on improving patient outcomes. OBJECTIVE This article aims to develop However, presentation can be variable – from indolent a structured approach to the investigation of patients with markers of kidney disease, and and asymptomatic to explosive with rapid loss of kidney promote the recognition of patients who need function. Pathology may be localised to the kidney or further assessment. Consideration is given to the part of a systemic illness. Therefore diagnosis involves importance of general measures required in the a systematic approach using a combination of clinical care of patients with GN. features, directed laboratory and radiological testing, DISCUSSION Glomerulonephritis is not an and in many (but not all) cases, a kidney biopsy to everyday presentation, however recognition establish the histological diagnosis. Management of and appropriate management is important to glomerulonephritis (GN) involves specific therapies prevent loss of kidney function. Disease specific directed at the underlying, often immunological cause treatment of GN may require specialist care, of the disease and more general strategies aimed at however much of the management involves delaying progression of kidney impairment. -
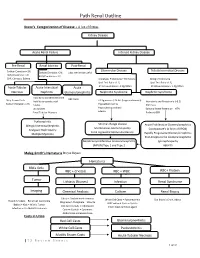
Path Renal Outline
Path Renal Outline Krane’s Categorization of Disease + A lot of Extras Kidney Disease Acute Renal Failure Intrinsic Kidney Disease Pre‐Renal Renal Intrinsic Post‐Renal Sodium Excretion <1% Glomerular Disease Tubulointerstitial Disease Sodium Excretion < 1% Sodium Excretion >2% Labs aren’t that useful BUN/Creatinine > 20 BUN/Creatinine < 10 CHF, Cirrhosis, Edema Urinalysis: Proteinuria + Hematuria Benign Proteinuria Spot Test Ratio >1.5, Spot Test Ratio <1.5, Acute Tubular Acute Interstitial Acute 24 Urine contains > 2.0g/24hrs 24 Urine contains < 1.0g/24hrs Necrosis Nephritis Glomerulonephritis Nephrotic Syndrome Nephritic Syndrome Inability to concentrate Urine RBC Casts Dirty Brown Casts Inability to secrete acid >3.5g protein / 24 hrs (huge proteinuria) Hematuria and Proteinuria (<3.5) Sodium Excretion >2% Edema Hypoalbuminemia RBC Casts Hypercholesterolemia Leukocytes Salt and Water Retention = HTN Focal Tubular Necrosis Edema Reduced GFR Pyelonephritis Minimal change disease Allergic Interstitial Nephritis Acute Proliferative Glomerulonephritis Membranous Glomerulopathy Analgesic Nephropathy Goodpasture’s (a form of RPGN) Focal segmental Glomerulosclerosis Rapidly Progressive Glomerulonephritis Multiple Myeloma Post‐Streptococcal Glomerulonephritis Membranoproliferative Glomerulonephritis IgA nephropathy (MPGN) Type 1 and Type 2 Alport’s Meleg‐Smith’s Hematuria Break Down Hematuria RBCs Only RBC + Crystals RBC + WBC RBC+ Protein Tumor Lithiasis (Stones) Infection Renal Syndrome Imaging Chemical Analysis Culture Renal Biopsy Calcium -

Nephrology Clinical Undergraduate Training
School of Medicine Nephrology clinical undergraduate training All care is taken to ensure that the information in this handbook is correct at the time of going to print. Handbook Version 5.0 Date of Origin: March 2019 Contents OBJECTIVES OF ATTACHMENT ................................................................................................... 3 TEACHING STRUCTURE: 3RD MEDICAL YEAR CLINICAL MEDICINE ATTACHMENTS .......................... 3 PROPOSED ATTACHMENT TIMETABLE: ................................................................................................ 5 READING LIST AND WEBSITES ............................................................................................................. 6 2 3rd Medical Year SPECIALTY: Nephrology - www.tcd.ie/medicine/thkc/education/ CONSULTANT: Prof G. Mellotte, Prof C Wall, Dr P Lavin, Dr B Griffin, Prof M Little HOSPITAL: Trinity Health Kidney Centre, Tallaght Hospital YEAR OF COURSE: 3 OBJECTIVES OF ATTACHMENT During this attachment, a student is expected to understand: - The clinical presentation of renal disease, e.g., proteinuria, hypertension, haematuria and uraemia. - Normal regulation of body water and sodium by the RAAS and ADH and how abnormalities give rise to changes in water and sodium homeostasis - Normal values of electrolytes in blood and urine and the clinical sequelae of a derangement in these. - A basic understanding of the following conditions: a. Acute kidney injury (pre-renal, post renal or intrinsic renal) b. Chronic kidney disease, focusing on diabetic nephropathy c. Glomerulonephritis: nephrotic / nephritic syndrome d. Myeloma and the kidney - The management of acute and chronic renal failure, including preparation for dialysis. - Impact of renal failure on drug handling. Be able to: - Take a full and appropriate current and past medical history. - Construct a synopsis or problem list based on the clinical assessment of a patient - Discuss the range of clinical investigations available and understand how they may be used to inform the differential diagnosis. -

16 the Kidney J
16 The Kidney J. Charles Jennette FPO FPO FPO FPO CONGENITAL ANOMALIES IgA Nephropathy (Berger Disease) Renal Agenesis Anti-Glomerular Basement Membrane Ectopic Kidney Glomerulonephritis Horseshoe Kidney ANCA Glomerulonephritis Renal Dysplasia VASCULAR DISEASES CONGENITAL POLYCYSTIC KIDNEY DISEASES Renal Vasculitis Autosomal Dominant Polycystic Kidney Disease Hypertensive Nephrosclerosis (Benign Nephrosclerosis) (ADPKD) Malignant Hypertensive Nephropathy Autosomal Recessive Polycystic Kidney Disease Renovascular Hypertension (ARPKD) Thrombotic Microangiopathy Nephronophthisis–Medullary Cystic Disease Cortical Necrosis ACQUIRED CYSTIC KIDNEY DISEASE DISEASES OF TUBULES AND INTERSTITIUM GLOMERULAR DISEASES Acute Tubular Necrosis (ATN) Nephrotic Syndrome Pyelonephritis Nephritic Syndrome Analgesic Nephropathy Glomerular Inflammation and Immune Mechanisms Drug-Induced (Hypersensitivity) Acute Tubulointerstitial Minimal-Change Glomerulopathy Nephritis Focal Segmental Glomerulosclerosis (FSGS) Light-Chain Cast Nephropathy Membranous Glomerulopathy Urate Nephropathy Diabetic Glomerulosclerosis RENAL STONES (NEPHROLITHIASIS AND Amyloidosis UROLITHIASIS) Hereditary Nephritis (Alport Syndrome) OBSTRUCTIVE UROPATHY AND HYDRONEPHROSIS Thin Glomerular Basement Membrane Nephropathy RENAL TRANSPLANTATION Acute Postinfectious Glomerulonephritis MALIGNANT TUMORS OF THE KIDNEY Type I Membranoproliferative Glomerulonephritis Wilms’ Tumor (Nephroblastoma) Type II Membranoproliferative Glomerulonephritis Renal Cell Carcinoma (RCC) (Dense Deposit Disease) -

Jemds.Com Original Research Article
Jemds.com Original Research Article CLINICAL PROFILE AND SHORT-TERM OUTCOME OF ACUTE NEPHRITIC SYNDROME IN CHILDREN Surya Kandashamparambil Kamalakarababu1, Ansu Sam2, Sajini Varghese3 1Assistant Professor, Department of Paediatrics, Government Medical College, Kottayam. 2Senior Resident, Department of Paediatrics, Government Medical College, Kottayam. 3Assistant Professor, Department of Paediatrics, Government Medical College, Kottayam. ABSTRACT BACKGROUND Glomerulonephritis generally presents as a constellation of findings that includes haematuria, proteinuria and oedema. Poststreptococcal glomerulonephritis is the commonest form of acute glomerulonephritis in developing countries. Objectives- 1. To study the clinical profile of acute glomerulonephritis. 2. To study the different clinical laboratory parameters at admission and at 8 weeks of onset of illness. MATERIAL AND METHODS This is a prospective, descriptive study conducted in a tertiary care teaching hospital in South India from January 2016-December 2016. Data regarding the clinical features, laboratory parameters, treatment were collected. All these patients were further followed up at 8 weeks and descriptive analysis done. RESULTS The most common cause of glomerulonephritis in the study group was poststreptococcal glomerulonephritis (42.9%) followed by drug-induced nephropathy (28.6%). The most common complaints were haematuria, facial puffiness, decreased urine output and oedema. Hypertension was found only in 14.3% of study group. 4.8% of patients had nephrotic range of proteinuria. The most common infection preceding was Pyoderma (61.5%) in case of PSGN. High ASO titre was seen commonly with pharyngitis. 45.2% patients had decreased Serum C3 at diagnosis. All the patients were treated conservatively. At 8 weeks followup, haematuria was persisting in 28.6% patients. Only 2 patients had persistently low serum C3 and single patient had high creatinine. -

A Guide for Primary Care Physicians
OPTIMUM PHYSICIAN ALLIANCE CONTACT PO Box 90 MERI NOTARO Buffalo, New York opawny.com 14240 1-844-769-5879 OPA 2017 NETWORK + INCENTIVE GUIDE Prepared For: Date Issued: A GUIDE FOR 10.31.2016 PRIMARY CARE PHYSICIANS Valid To: 12.31.2017 OPA PHYSICIAN PARTNERS Table of Contents CONTENTS 1 2 3 Take a look at what you'll find inside this guide. INTRODUCTION TIERING PROGRAM STANDARD INCENTIVE OPA Overview 3 OPA Primary Care 12 Standard Incentive Overview 17 Physician Tiering Overview 2017 Network Standards 5 Primary Care Metrics 19 2017 PCP 13 OPA Resources 7 Tiering Structure Pediatric Care Metrics 22 Network Incentive and 9 Standard Incentive Metrics 23 PCP Tiering Overview 4 5 6 CENTERS OF EXCELLENCE PRACTICE OPTIMIZATION APPENDICES PROGRAM (POP) Centers of Excellence 25 Practice Optimization 31 Standard Incentive 45 Overview Program (POP) Guidelines Code Lists Activity Based 27 Sample POP Application 39 Performance Measures Utilization Based 28 Performance Measures Centers of Excellence Metrics 29 1 OPTIMUM PHYSICIAN ALLIANCE OPTIMUM PHYSICIAN ALLIANCE 2 [ the platform that simplifies the practice of medicine ] Optimum Physician Alliance (OPA), formed in 2012, is the platform that simplifies the practice of medicine. It consists of approximately 600 physicians in the areas of primary and specialty care, Organization located mainly in Erie and Niagara counties. OPA is committed to promoting prac- meaningful engagement among healthcare tice transformation, with a patient-centered partners. OPA provides key resources to reduce Overview approach, and understands the importance administrative burdens and offers incentive pro- of leveraging healthcare data to support high grams designed to promote the type of practice quality outcomes for patients. -

Download Download
Mauskar et al Inhalation Steroids and Nephrotic Syndrome Doi: 10.32677/IJCR.2015.v01.i03.001 Case Series Effect of Inhalation Steroids in Children with Coexistent Atopic Disorders & Nephrotic Syndrome on Prevention of Relapses of Nephrotic Syndrome Anupama Mauskar, Geeta Mandhani, Pawan Deore From, Department Of Pediatrics, Lokmanya Tilak Municipal Medical College and General Hospital, Mumbai. Correspondence to: Dr. Anupama Mauskar, 401, ‘UTTUNG’ CHSD. L Vaidya Road, Dadar (West) Mumbai - 400028, E- mail – [email protected] Received: 23 September 2015 Initial Review: 13 October 2015 Accepted: 22 November 2015 Published Online: 13 December 2015 ABSTRACT In many nephrotic syndrome children, it has been observed that onset of rhinitis and/or wheezing attacks precipitate the relapse of nephrotic syndrome. This association is known since long time. We decided to observe the response of inhalation steroids in prevention of relapses of nephrotic syndrome in children who presented with frequently relapsing nephrotic syndrome and coexistent atopic disorders. Key words: Atopy, Inhalation steroids, Nephrotic syndrome, Relapse ssociation of idiopathic nephrotic syndrome and an episode of exacerbation of wheezing or rhinitis. The atopic disorders has been observed by fanconi et frequency of relapses varied from 3-8/ year. A al for the first time in 1951 and then by many observers [1-3]. Whether it is a type of allergic disorder, These were treated with oral prednisolone 2mg.kg/day the evidence is scarce [4]. We share our experience of till remission and 1.5mg/kg/alternate day for 4 weeks seven patients with asthma and rhinitis who also had along with the treatment of underlying infection. -

Glomerular Diseases Associated with Malignancies: Histopathological Pattern and Association with Circulating Autoantibodies
antibodies Article Glomerular Diseases Associated with Malignancies: Histopathological Pattern and Association with Circulating Autoantibodies Sophia Lionaki 1,* , Smaragdi Marinaki 1, Konstantinos Panagiotellis 1, Ioanna Tsoumbou 1, George Liapis 2, Ioanna Vlahadami 3, Athanasios Tzioufas 3, Petros Sfikakis 4 and Ioannis Boletis 1 1 Nephrology Department & Transplantation Unit, Faculty of Medicine, Laiko Hospital, National & Kapodistrian University of Athens, 11527 Athens, Greece; [email protected] (S.M.); [email protected] (K.P.); [email protected] (I.T.); [email protected] (I.B.) 2 Pathology Department, Faculty of Medicine, Laiko Hospital, National & Kapodistrian University of Athens, 11527 Athens, Greece; [email protected] 3 Department of Pathophysiology, Faculty of Medicine, Laiko Hospital, National & Kapodistrian University of Athens, 11527 Athens, Greece; [email protected] (I.V.); [email protected] (A.T.) 4 1st Department of Propaedeutic and Internal Medicine and Joined Rheumatology Program, Medical School, Laiko Hospital, National and Kapodistrian University of Athens, 11527 Athens, Greece; psfi[email protected] * Correspondence: sofi[email protected]; Tel.: +30-2110195670; Fax: +30-2132061856 Received: 31 March 2020; Accepted: 18 May 2020; Published: 25 May 2020 Abstract: Aim: Glomerular diseases (GD) associated with malignancies (AM, GDAM) have unique features, which are important to recognize, in the light of the progress made in cancer therapy. We aimed to describe the clinical and histopathological characteristics of patients with GDAM in relation to the presence of circulating autoantibodies, pointing to potential immune pathogenic pathways connecting cancer to GD. Materials and Methods: The included patients were studied retrospectively on the basis of a kidney biopsy proving GD and a related biopsy to establish the diagnosis of AM. -

Redalyc.Nephritic Syndrome Associated to Skin Infection
Colombia Médica ISSN: 0120-8322 [email protected] Universidad del Valle Colombia Barrios, Emil Julio; Guerrero, Gustavo Adolfo Nephritic syndrome associated to skin infection, hepatitis A, and pneumonia: a case report Colombia Médica, vol. 41, núm. 2, abril-junio, 2010, pp. 171-175 Universidad del Valle Cali, Colombia Available in: http://www.redalyc.org/articulo.oa?id=28316817009 How to cite Complete issue Scientific Information System More information about this article Network of Scientific Journals from Latin America, the Caribbean, Spain and Portugal Journal's homepage in redalyc.org Non-profit academic project, developed under the open access initiative Colombia Médica Vol. 41 Nº 2, 2010 (Abril-Junio) Nephritic syndrome associated to skin infection, hepatitis A, and pneumonia: a case report EMIL JULIO B ARRIOS, MD1, G USTAVO ADOLFO G UERRERO, MD2 SUMMARY Introduction: Glomerulonephritis is the most common cause of acute and chronic renal disease. The prototype of acute glomerulonephritis is acute post-infectious glomerulonephritis. Recently, increased cases of glomerulopathy have been associated with bacterial, viral, and other infections. Acute nephritic syndrome is part of glomerulonephritis with an acute beginning, characterized by hematuria, hypertension, edema, and oliguria due to the reduction of glomerular filtration reflected in an increase of nitrogen compounds. Development: This paper shows a male infant at 2 years and 7 months of age with nephritic syndrome associated to a skin infection, pneumonia, and hepatitis A virus infection. Conclusion: Acute glomerulonephritis may be associated to streptococcus or another coincidental infection. Children with skin infection, hepatitis A, or pneumonia who reveal abnormal urinalysis, hypertension, azotemia, or oliguria should be evaluated for concomitant glomerulonephritis. -

Nephrology 1
The University of Arizona Pediatric Residency Program Primary Goals for Rotation Nephrology 1. GOAL: Understand the general pediatrician's role in diagnosis and management of hypertension in children. 2. GOAL: Understand the role of the pediatrician in preventing renal disease, and in counseling and screening individuals at risk for these diseases. 3. GOAL: Differentiate between normal and pathological states related to the renal system. 4. GOAL: Evaluate, treat, and/or refer patients with presenting signs and symptoms that may indicate a nephrologic disease process. 5. GOAL: Diagnose and manage patients with common renal conditions who generally do not require referral. 6. GOAL: Recognize, initiate management of patients with renal conditions who generally require referral. 7. GOAL: Understand the physiology of body fluids and electrolytes, abnormalities, and treatment. 8. GOAL: Appropriately manage and refer, when necessary, patients with urinary tract infections. 9. GOAL: Understand the pediatrician's role in the management of nephrotic syndrome. 10. GOAL: Understand the pathophysiology and management of common systemic conditions that may present with renal involvement, and seek consultation or referral appropriately. 11. GOAL: Demonstrate high standards of professional competence while working with patients under the care of a subspecialist. 1. GOAL: Hypertension. Understand the general pediatrician's role in diagnosis and management of hypertension in children. A. Classify a patient with hypertension as to severity according to current national guidelines, e.g., mild, moderate or severe. B. Develop a diagnostic plan for a child with hypertension that accounts for severity of the condition, including recognition and management of hypertensive emergencies. C. Manage a patient with hypertension using a step-wise approach that includes the role of diet, exercise, weight control and medications. -

4 Part 1 the NEPHROTIC SYNDROME
بسم هللا الرحمن الرحيم After hours, or maybe days, of working hard, WE “THE PATHOLOGY TEAM” are proud to present “PATHOLOGY OF THE RENAL SYSTEM”, I hope you guys like it . Plz give us your prayers. Credits: 1st part = written by Assem “ THe AWesOme” Kalantan revised by A.Z.K 2nd part = written by TMA revised by A.Z.K د.خالد القرني 3rd part = written by Abo Malik revised by 4th part = written by A.Z.K revised by Assem “ THe AWesOme” Kalantan 5th part = written by The Dude revised by TMA figures were provided by A.Z.K Page styling and figure embedding by: If u find any error, or u want to share any idea then plz, feel free to msg me [email protected] Table of Contents 2 Topic page THE NEPHROTIC SYNDROME 4 Minimal Change Disease 5 MEMBRANOUS GLOMERULONEPHRITIS 7 FOCAL SEGMENTAL GLOMERULOSCLEROSIS 9 MEMBRANOPROLIFERATIVE 11 GLOMERULONEPHRITIS Nephritic Syndrome 16 Acute Postinfectious GN 17 IgA Nephropathy (Berger Disease) 18 Crescentic GN 20 Chronic GN 21 Urinary Tract OBSTRUCTION, 23 RENAL STONES 23 HYDRONEPHROSIS 25 ACUTE POLYNEPHRITIS 27 CHRONIC PYELONEPHRITIS 30 DRUG-INDUCED Interstitial Nephritis 32 Tumors of the kidney and urinary 33 bladder Benign tumors 33 Clear Cell Carcinomas 34 Papillary Renal Cell Carcinoma 34 Chromophope Renal Carcinomas 35 Wilms'Tumor 37 WAGR syndrome, Denys-Darsh syndrome 37 Beckwith-Wiedemann syndrome 38 Tumors of the Urinary Bladder and Collecting 40 System 3 part 1 THE NEPHROTIC SYNDROME refers to a clinical complex that includes the following: (1) massive proteinuria - daily loss of 3.5 or more in the urine (2) hypoalbuminemia - plasma albumin levels less than 3 g/dL (3) generalized edema - the most obvious clinical manifestation (4) hyperlipidemia and lipiduria The initial event is a derangement in the capillary walls of the glomeruli, resulting in increased permeability to the plasma proteins In the normal kidney - the glomerular capillary wall, with its endothelium, basement membrane (GBM), and podocytes , acts as a barrier through which the glomerular filtrate must pass.