Note Formation of an Inclusion Complex of Anthocyanin With
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
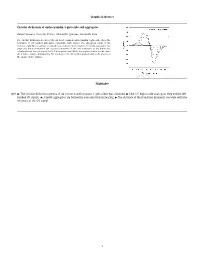
Graphical Abstract Circular Dichroism of Anthocyanidin 3-Glucoside Self
Graphical abstract Circular dichroism of anthocyanidin 3-glucoside self-aggregates Raquel Gavara, Vesselin Petrov, Alexandre Quintas, Fernando Pina * The circular dichroism spectra of the six most common anthocyanidin 3-glucoside show the formation of left handed aggregates compatible with dimers. The absorption bands of the monomer split by increasing concentration according to the formation of H and J aggregates. The angle and distance between the transition moments of the two monomers in the dimer was calculated from the splitting of the 0–0 absorption band. While the angle is similar for the series the distance changes dramatically. The intensity of the CD signal is proportional to the inverse of the square of the distance. Highlights Q10 " The circular dichroism spectra of six common anthocyanins 3-glucosides was obtained. " Like 3,5-diglucoside analogous they exhibit left- handed CD signals. " J and H aggregates are formed by concentration increasing. " The distance of the transition moments correlate with the intensity of the CD signal. 1 1 2 Circular dichroism of anthocyanidin 3-glucoside self-aggregates a a b a,⇑ 3 Q1 Raquel Gavara , Vesselin Petrov , Alexandre Quintas , Fernando Pina 4 a REQUIMTE, Departamento de Química, Faculdade de Ciências e Tecnologia, Universidade Nova de Lisboa, 2829516 Monte de Caparica, Portugal 5 b Instituto Superior de Ciencias da Saude Egas Moniz, Centro de Investigação Interdisciplinar Egas Moniz, P-2829511 Monte de Caparica, Caparica, Portugal 6 7 article info a b s t r a c t 1 9 10 Self-association constants for the flavylium cations of the six most common anthocyanidin 3-glucosides 20 11 were determined by circular dichroism (CD) and UV–Vis spectroscopy. -

Electrochemical and Antioxidant Properties of Anthocyanins and Anthocyanidins
CROATICA CHEMICA ACTA CCACAA 80 (1) 29¿34 (2007) ISSN-0011-1643 CCA-3135 Original Scientific Paper Electrochemical and Antioxidant Properties of Anthocyanins and Anthocyanidins Andréia A. de Lima, Eliana M. Sussuchi, and Wagner F. De Giovani* Departamento de Química, Faculdade de Filosofia, Ciências e Letras de Ribeirão Preto, Universidade de São Paulo, Av. Bandeirantes 3900, 14040-901, Ribeirão Preto – SP, Brazil RECEIVED JUNE 26, 2006; REVISED SEPTEMBER 29, 2006; ACCEPTED OCTOBER 27, 2006 Electrochemical properties of delphinidin, cyanidin, pelargonidin, kuromanin and callistephin were investigated by cyclic and differential pulse voltammetries at different pH values and also in methanol. On the basis of oxidation potentials, the order of antioxidant activity for antho- cyanidins is delphinidin > cyanidin > pelargonidin. Oxidation peaks for anthocyanins (kuro- manin and callistephin) are shifted to more positive potentials compared to anthocyanidins (delphinidin, cyanidin and pelargonidin). Oxidation peak currents are linearly dependent on the Keywords square root of the scan rate, which is typical of a diffusion controlled electrochemical process. anthocyanins Antioxidant activities of the compounds were evaluated using the 1,1-diphenyl-2-picrylhydra- anthocyanidins zyl (DPPH) radical-scavenging method and they are directly related to their redox potential antioxidants values. The order of the antioxidant activity is delphinidin > cyanidin > pelargonidin > kuro- electrochemical properties manin > callistephin. INTRODUCTION pharmacological properties that render them interesting potential cancer chemopreventive agents.9–11 Because Anthocyanins are one of the main classes of flavonoids anthocyanins are widely consumed, finding out addi- in red wines. They have been pointed out as the molecu- tional biological activities related to these compounds lar entities that are most likely responsible for what has would be of great interest. -

A Combined Experimental and Computational Study of Chrysanthemin As a Pigment for Dye-Sensitized Solar Cells
molecules Article A Combined Experimental and Computational Study of Chrysanthemin as a Pigment for Dye-Sensitized Solar Cells Atoumane Ndiaye 1,2 , Alle Dioum 1, Corneliu I. Oprea 2 , Anca Dumbrava 3,* , Jeanina Lungu 2, Adrian Georgescu 2, Florin Moscalu 2, Mihai A. Gîr¸tu 2,* , Aboubaker Chedikh Beye 1 and Issakha Youm 1,* 1 Department of Physics, Cheikh Anta Diop University of Dakar, 5005 Dakar-Fann, Senegal; [email protected] (A.N.); [email protected] (A.D.); [email protected] (A.C.B.) 2 Department of Physics, Ovidius University of Constanta, 900527 Constanta, Romania; [email protected] (C.I.O.); [email protected] (J.L.); [email protected] (A.G.); fl[email protected] (F.M.) 3 Department of Chemistry and Chemical Engineering, Ovidius University of Constanta, 900527 Constanta, Romania * Correspondence: [email protected] (A.D.); [email protected] (M.A.G.); [email protected] (I.Y.) Abstract: The theoretical study of chrysanthemin (cyanidin 3-glucoside) as a pigment for TiO2-based dye-sensitized solar cells (DSSCs) was performed with the GAUSSSIAN 09 simulation. The electronic spectra of neutral and anionic chrysanthemin molecules were calculated by density functional theory with B3LYP functional and DGDZVP basis set. A better energy level alignment was found for partially deprotonated molecules of chrysanthemin, with the excited photoelectron having enough energy in order to be transferred to the conduction band of TiO2 semiconductor in DSSCs. In addition, we used the raw aqueous extracts of roselle (Hibiscus sabdariffa) calyces as the source of chrysanthemin Citation: Ndiaye, A.; Dioum, A.; and the extracts with various pH values were tested in DSSCs. -

Callistephin Enhances the Protective Effects of Isoflurane on Microglial Injury Through Downregulation of Inflammation and Apoptosis
802 MOLECULAR MEDICINE REPORTS 20: 802-812, 2019 Callistephin enhances the protective effects of isoflurane on microglial injury through downregulation of inflammation and apoptosis LILI ZHAO, SHIBIAO CHEN, TIANYIN LIU, XIUHONG WANG, HAIJIN HUANG and WEICHENG LIU Department of Anesthesiology, The First Affiliated Hospital of Nanchang University, Nanchang, Jiangxi 330006, P.R. China Received June 18, 2018; Accepted March 15, 2019 DOI: 10.3892/mmr.2019.10282 Abstract. Microglia are the major immune cells in the central enhanced the effects of isoflurane. Callistephin may therefore nervous system. Microglial activation can be beneficial or constitute a candidate drug agent that may target inflammatory detrimental depending on the stimuli and the physiopathological and growth regulatory signaling pathways, thus ameliorating environment. Microglial activation is involved in a variety certain aspects of neurodegenerative diseases. of neurodegenerative disorders. Different anesthetic agents have exhibited diverse effects on microglial activation and Introduction the engulfment process. The anthocyanin callistephin has been demonstrated to have antioxidant and anti‑inflammatory Microglial cells are the major immune cell in the central properties, and these were assessed in the present study, with a nervous system (CNS), responding against types of endog- focus on its effect on microglial activation. Mouse microglial enous and exogenous stimuli, including infection by bacteria, cells C8-4B were treated with 100 ng/µl lipopolysaccharide viruses, prions and β-amyloid plaques (1). Microglia are (LPS) and 1 ng/µl interferon-γ. Cells were subsequently treated activated upon exposure to different stimuli and, depending with 2% isoflurane, 100 µM callistephin or both. LPS promoted on the environmental context, this may be beneficial or detri- apoptosis in C8-B4 cells, and this was reduced following mental to the functionality and physiology of the CNS (2). -

Characterisation of Bioactive Compounds in Berries from Plants Grown Under Innovative Photovoltaic Greenhouses
Journal of Berry Research 8 (2018) 55–69 55 DOI:10.3233/JBR-170258 IOS Press Characterisation of bioactive compounds in berries from plants grown under innovative photovoltaic greenhouses Federica Blandoa,∗, Carmela Gerardia, Massimiliano Rennab,c, Sergio Castellanod and Francesco Seriob aInstitute of Sciences of Food Production (ISPA), CNR, Lecce, Italy bInstitute of Sciences of Food Production (ISPA), CNR, Bari, Italy cDepartment of Agricultural and Environmental Science, University of Bari Aldo Moro, Bari, Italy dDepartment of Science of Agriculture, Food and Environment, (SAFE) University of Foggia, Foggia, Italy Received 2 July 2017; accepted 1 November 2017 Abstract. BACKGROUND: Bioactive compounds, mainly polyphenols, present in berries, are thought to be responsible for the health benefits of these fruit. Therefore, it is worthwhile to define the optimal environmental conditions to maximise their polyphenol content. OBJECTIVE: With the aim to define the optimal conditions for berry cultivation in an innovative environment, red rasp- berry, wild strawberry and blackberry plants were grown in a traditional greenhouse in comparison with two photovoltaic greenhouses with different shading area. METHODS: Hydroalcoholic extracts of ripe berries were evaluated by HPLC analysis, for their anthocyanins, organic acids and sugar contents. Moreover, phenolic content (by the Folin-Ciocalteu assay) and antioxidant activity (by the Trolox equivalent antioxidant capacity-TEAC assay) were assayed on the same berry extracts. RESULTS: Total anthocyanins, -

Effects of Anthocyanins on the Ahr–CYP1A1 Signaling Pathway in Human
Toxicology Letters 221 (2013) 1–8 Contents lists available at SciVerse ScienceDirect Toxicology Letters jou rnal homepage: www.elsevier.com/locate/toxlet Effects of anthocyanins on the AhR–CYP1A1 signaling pathway in human hepatocytes and human cancer cell lines a b c d Alzbeta Kamenickova , Eva Anzenbacherova , Petr Pavek , Anatoly A. Soshilov , d e e a,∗ Michael S. Denison , Michaela Zapletalova , Pavel Anzenbacher , Zdenek Dvorak a Department of Cell Biology and Genetics, Faculty of Science, Palacky University, Slechtitelu 11, 783 71 Olomouc, Czech Republic b Institute of Medical Chemistry and Biochemistry, Faculty of Medicine and Dentistry, Palacky University, Hnevotinska 3, 775 15 Olomouc, Czech Republic c Department of Pharmacology and Toxicology, Charles University in Prague, Faculty of Pharmacy in Hradec Kralove, Heyrovskeho 1203, Hradec Kralove 50005, Czech Republic d Department of Environmental Toxicology, University of California, Meyer Hall, One Shields Avenue, Davis, CA 95616-8588, USA e Institute of Pharmacology, Faculty of Medicine and Dentistry, Palacky University, Hnevotinska 3, 775 15 Olomouc, Czech Republic h i g h l i g h t s • Food constituents may interact with drug metabolizing pathways. • AhR–CYP1A1 pathway is involved in drug metabolism and carcinogenesis. • We examined effects of 21 anthocyanins on AhR–CYP1A1 signaling. • Human hepatocytes and cell lines HepG2 and LS174T were used as the models. • Tested anthocyanins possess very low potential for food–drug interactions. a r t i c l e i n f o a b s t r a c t -

First-Pass Metabolism of Polyphenols from Selected Berries: a High-Throughput Bioanalytical Approach
antioxidants Article First-Pass Metabolism of Polyphenols from Selected Berries: A High-Throughput Bioanalytical Approach Francisco J. Olivas-Aguirre 1,*, Sandra Mendoza 2, Emilio Alvarez-Parrilla 3 , Gustavo A. Gonzalez-Aguilar 4 , Monica A. Villegas-Ochoa 4 , Jael T.J. Quintero-Vargas 1 and Abraham Wall-Medrano 3,* 1 Departamento de Ciencias de la Salud, Universidad de Sonora (Campus Cajeme), Blvd Bordo Nuevo s/n, Ejido Providencia, Cd, Obregón 85199, Mexico; [email protected] 2 Departamento de Investigación y Posgrado en Alimentos (PROPAC), Facultad de Química, Universidad Autónoma de Querétaro, Cerro de las Campanas s/n, Santiago de Querétaro 76010, Mexico; [email protected] 3 Instituto de Ciencias Biomédicas, Universidad Autónoma de Ciudad Juárez, Anillo Envolvente del PRONAF y Estocolmo s/n, Ciudad Juárez 32310, Mexico; [email protected] 4 Centro de Investigación en Alimentación y Desarrollo, A.C. (CIAD), Carretera a Ejido La Victoria, Km. 0.6, Hermosillo 83304, Mexico; [email protected] (G.A.G.-A.); [email protected] (M.A.V.-O.) * Correspondence: [email protected] (F.J.O.-A.); [email protected] (A.W.-M.) Received: 29 March 2020; Accepted: 11 April 2020; Published: 13 April 2020 Abstract: Small berries are rich in polyphenols whose first-pass metabolism may alter their ultimate physiological effects. The antioxidant capacity and polyphenol profile of three freeze-dried berries (blackberry, raspberry, Red Globe grape) were measured and their apparent permeability (Papp) and first-pass biotransformation were tracked with an ex vivo bioanalytical system [everted gut sac (rat) + three detection methods: spectrophotometry, HPLC-ESI-QTOF-MS, differential pulse voltammetry (DPV)]. -

Compositions of Anthocyanin and Other Flavonoids in Cultured Cells of Rabbiteye Blueberry (Vaccinium Ashei Reade Cv
Food Sci. Technol Res., 10 (3), 239-246, 2004 Compositions of Anthocyanin and Other Flavonoids in Cultured Cells of Rabbiteye Blueberry (Vaccinium ashei Reade cv. Tiiblue) Shioka HAMAMATSU,Kimiko YABE and Yoshihiko NAWA National Food Research Institute, 2-1-12 Kannondai, Tsukuba, Ibaraki 305-8642, Japan Received August 18, 2003 ; Accepted April 2, 2004 Our blueberry cultured cells produce anthocyanins in high quantity (Nawa et al., Biosci. Biotech. Biochem., 57, 770-774, 1993). Using high performance liquid chromatography/mass spectrometry (LC-MS), 14 anthocyanins in the red cells derived from leaves of rabbiteye blueberry cv. Tifblue (Vaccinium ashei Reade) were identified as 3-galactoside (gal), 3-glucoside (glc) and 3-arabinoside (ara) of cyanidin (Cy), delphinidin (Dp), petunidin (Pe), peonidin (Pn) and malvidin (Mv), except for Mv 3-ara. The fruits additionally contained 15 anthocyanins and Mv 3-ara, as a main anthocyanin. The autumn red leaves contained Cy 3-gal, Cy 3-ara and Cy 3-glc. In the red cells and the leaves, derivatives of Cy were the most abundant at 85.7% (Cy 3-gal 49.1%, Cy 3-glc 5.8%, Cy 3-ara 30.9%) and 100% (Cy 3-gal 51.0%, Cy 3-glc 6.5%, Cy 3-ara 42.5%) of the total anthocyanins, respectively. In contrast, the fruits contained Mv derivatives as main components that amounted to 50.9% of the total anthocyanins; Mv 3-gal, Mv 3-ara and Mv 3-glc constituted 21.2, 15.3 and 14.4%, respectively. Cy derivatives in fruit anthocyanins amounted to 18.0% . The red cells produced 107 mg of anthocyaninlIOO g fr wt, which was slightly lower than that of the fruits (129 mg). -

A Biochemical Survey of Some Mendelian Factors for Flower Colour
A BIOCHEMICAL 8UP~VEY OF SOME MENDELIAN FACTOI%S FO].~ FLOWEP~ COLOU~. BY ROSE SCOTT-MONCI~IEFF. (John Inncs Horticultural Institution, London.) (With One Text-figure.) CONTENTS. PAGE P~rb I. Introductory ].17 (a) The plastid 1)igmenl~s ] 21 (b) The a,n~hoxan~hius: i~heir backgromld, co-pigment and interaction effecbs upon flower-colour v~ri~bion 122 (c) The ani~hocyauins ] 25 (c) Col[oidM condition . 131 (f) Anthoey~nins as indic~bors 132 (g) The source of tim ~nl;hoey~nins 133 ]?ar[ II, Experimental 134 A. i~ecen~ investigations: (a) 2Prim,ula si,sensis 134- (b) Pa,l)aver Rhoeas 14.1 (c) Primuln aca.ulis 147 (d) Chc.l)ranth'ss Chci,rl 148 (e) ltosa lmlyanlha . 149 (f) Pelargonium zomdc 149 (g) Lalh,ymts odor~,l,us 150 (h) Vcrbom, hybrids 153 (i) Sl;'e2)loca~'])uG hybrids 15~ (j) T'rol)aeolu,m ,majors ] 55 ]3. B,eviews of published remflts of bhe t~u~horand o~hers.. (a) Dahlia variabilis (Lawreuce and Scol,~-Monerieff) 156 (b) A.nlb'rhinum majors (Wheklalo-Onslow, :Basseb~ a,nd ,~cobb- M.oncrieff ) 157 (c) Pharbilis nil (I-Iagiwam) . 158 (d) J/it& (Sht'itl.er it,lid Anderson) • . 159 (e) Zect d]f.ctys (~&udo, Miiner trod 8borl/lall) 159 Par~, III. The generM beh~wiour of Mendelian £acbors rot' flower colour . 160 Summary . 167 tLefermmes 168 I)AI~T I. II~TI~O])UOTOnY. Slm~C~ Onslow (1914) m~de the first sfudy of biochemica] chal~ges in- volved in flower-eolour va,riadon, our pro'ely chemical knowledge of bhe 118 A Bio&emical Su~'vey oI' Factor's fo~ • Flowe~' Colou~' anthocya.nin pigments has been considerably advanced by the work of Willstgtter, P~obinson, Karrer and their collaborators. -

Cyanidin-3-O-Glucoside: Physical-Chemistry, Foodomics and Health Effects
molecules Review Cyanidin-3-O-glucoside: Physical-Chemistry, Foodomics and Health Effects Francisco J. Olivas-Aguirre 1, Joaquín Rodrigo-García 1, Nina del R. Martínez-Ruiz 1, Arely I. Cárdenas-Robles 2, Sandra O. Mendoza-Díaz 2, Emilio Álvarez-Parrilla 1, Gustavo A. González-Aguilar 3, Laura A. de la Rosa 1, Arnulfo Ramos-Jiménez 1 and Abraham Wall-Medrano 1,* 1 Instituto de Ciencias Biomédicas, Departamento de Ciencias Químico-Biológicas, Universidad Autónoma de Ciudad Juárez, Anillo Envolvente del PRONAF y Estocolmo s/n, Ciudad Juárez 32310, Chihuahua, Mexico; [email protected] (F.J.O.-A.); [email protected] (J.R.-G.); [email protected] (N.d.R.M.-R.); [email protected] (E.Á.-P.); [email protected] (L.A.d.l.R.); [email protected] (A.R.-J.) 2 Departamento de Investigación y Posgrado en Alimentos, Facultad de Química, Universidad Autónoma de Querétaro, Cerro de las Campanas s/n, Querétaro 76010, Querétaro, Mexico; [email protected] (A.I.C.-R.); [email protected] (S.O.M.-D.) 3 Coordinación de Tecnología de Alimentos de Origen Vegetal, Centro de Investigación en Alimentación y Desarrollo, AC. Carretera a la Victoria km. 0.6, AP 1735, Hermosillo 83000, Sonora, Mexico; [email protected] * Correspondence: [email protected]; Tel.: +52-(656)-688-1821; Fax: +52-(656)-688-1800 Academic Editors: Celestino Santos-Buelga and Arturo San Feliciano Received: 15 August 2016; Accepted: 13 September 2016; Published: 21 September 2016 Abstract: Anthocyanins (ACNs) are plant secondary metabolites from the flavonoid family. Red to blue fruits are major dietary sources of ACNs (up to 1 g/100 g FW), being cyanidin-3-O-glucoside (Cy3G) one of the most widely distributed. -

Color and Pigment Analyses in Fruit Products
Station Bulletin 624 Reprinted May 1993 Color and Pigment Analyses in Fruit Products Agriculiural Experiment Station Oregon State University For additional copies of this publication, write: Publications Orders Agricultural Communications Oregon State University Administrative Services A422 Corvallis, OR 97331-2119 Agricultural Experiment Station Oregon State University Station Bulletin 624 Reprinted May 1993 Color and Pigment Analyses in Fruit Products Ronald E. Wroistad Professor of Food Science and Technology Oregon State University In July 1992 the warehouse storing Oregon Agricultural Experiment Station publications was destroyed by fire. SB 624, originally published in October, 1976, is hereby reprinted in its entirety. COLOR AND PIGMENT ANALYSES IN FRUIT PRODUCTS Ronald E. Wroistad ABSTRACT Methods are described for making the following deter- minations in fruits and processed fruit products: anthocyanin pigment content, color density, polymeric color, browning, turbidity, and anthocyanin degrada- tion index. Tables list the anthocyanins of common fruits, their molecular weights, molar absorbances, and wavelengths of maximum absorption. Thirty-one references are given. KEY WORDS: color analyses, anthocyanin pigment, browning, fruits Introduction Measurement of pigment content, browning, color density, and haze are analytical problems confronting the food technologist working with fruit concentrates, juices, wines, jams and other processedpro- ducts. This publication includes some of the methods we have found practical in determining these parameters. We also have included tables which list anthocyanin pigments of fruits likelyto be processed in the Northwest and the molar absorption of the common occurring anthocyanin pigments. We have not included procedures of pigment extraction, isolation, and identification or methods for tristimulus colorimetry.Tristimulus colorimeters, such as the Hunter color-difference meter and the Lovi- bond tintometer, give the best measurements for visualappearance of a product. -

Supplementary Materials
Supplementary Materials Table S1. Metals with stdev W1 W2 W3 W4 W5 W6 W7 W8 W9 W10 W11 W12 W13 As <LOD <LOD 19.84±0.03 <LOD <LOD <LOD <LOD <LOD <LOD <LOD <LOD <LOD <LOD Ba 32.6±0.3 33.8±0.4 26.1±0.2 123.3±0.9 79.8±0.8 79.1±0.7 83.3±0.6 239.7±0.8 58.1±0.4 77.7±0.3 32.1±0.3 31.7±0.5 122.1±0.8 Cd 0.365±0.004 1.553±0.002 0.939±0.002 1.254±0.003 0.386±0.005 1.367±0.008 0.733±0.004 0.050±0.002 0.976±0.002 1.644±0.006 <LOD 0.593±0.004 0.375±0.003 Co 3.64±0.04 2.09±0.02 0.98±0.03 4.40±0.06 2.77±0.02 1.83±0.02 2.42±0.02 2.68±0.03 7.34±0.09 7.78±0.07 2.12±0.04 1.61±0.02 2.60±0.03 Cr <LOD <LOD <LOD <LOD 4.322±0.005 3.030±0004 <LOD 6.417±0.006 15.41±0.02 14.82±0.05 11.12±0.04 9.15±0.02 3.111±0.004 Pb <LOD 56.0±0.8 <LOD 20.8±0.9 80±2 11.7±0.6 2.20±0.05 175±3 45.0±0.8 <LOD 0.830±0.007 0.538±0.006 14.9±0.3 Sb 32.8±0.5 8.59±0.09 19.1±0.2 18.±0.2 67.5±0.5 37.9±0.2 9.58±0.05 46.5±0.3 44.8±0.4 18.50±0.08 7.26±0.03 44.3±0.2 29.6±0.4 Se <LOD <LOD <LOD <LOD 1.45±0.09 0.30±0.05 0.23±0.08 25.7±0.4 2.87±0.2 2.06±0.04 2.91±0.8 2.38±0.07 2.99±0.06 Ni 4.17±0.05 46.0±0.2 0.569±0.005 30.9±0.3 15.3±0.2 5.77±0.02 35.2±0.3 13.86±0.04 50.6±0.7 17.9±0.2 9.99±0.03 0.197±0.007 5.00±0.02 Al 566±5 260.1±0.5 155.3±0.4 720±5 447±4 290.7±2 26.5±0.4 155.4±0.9 444±4 329±3 1027±10 567.7±8 236±3 Cu 97.5±0.8 469±3 36.3±0.9 10.07±0.05 <LOD <LOD <LOD <LOD 21.19±0.07 7.73±0.03 0.813±0.05 49.3±0.8 50.2±1.0 Mn 735±30 613±10 642±6 1016±6 762±3 743±5 839±8 1634±30 1732±20 1472±20 887±10 771±6 1299±10 V 4.18±0.02 1.894±0.007 0.574±0.002 <LOD 1.530±0.005 <LOD 1.948±0.006 3.179±0.004 3.235±0.007