日汉食品工业词汇/姬德衡,朱蓓薇主编.—2 版.—北京: 中国轻工业出版社,2008.2 Isbn 978-7-5019-6314-0 日汉食品工业词汇 ⅰ
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
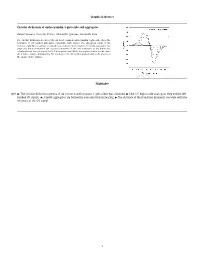
Graphical Abstract Circular Dichroism of Anthocyanidin 3-Glucoside Self
Graphical abstract Circular dichroism of anthocyanidin 3-glucoside self-aggregates Raquel Gavara, Vesselin Petrov, Alexandre Quintas, Fernando Pina * The circular dichroism spectra of the six most common anthocyanidin 3-glucoside show the formation of left handed aggregates compatible with dimers. The absorption bands of the monomer split by increasing concentration according to the formation of H and J aggregates. The angle and distance between the transition moments of the two monomers in the dimer was calculated from the splitting of the 0–0 absorption band. While the angle is similar for the series the distance changes dramatically. The intensity of the CD signal is proportional to the inverse of the square of the distance. Highlights Q10 " The circular dichroism spectra of six common anthocyanins 3-glucosides was obtained. " Like 3,5-diglucoside analogous they exhibit left- handed CD signals. " J and H aggregates are formed by concentration increasing. " The distance of the transition moments correlate with the intensity of the CD signal. 1 1 2 Circular dichroism of anthocyanidin 3-glucoside self-aggregates a a b a,⇑ 3 Q1 Raquel Gavara , Vesselin Petrov , Alexandre Quintas , Fernando Pina 4 a REQUIMTE, Departamento de Química, Faculdade de Ciências e Tecnologia, Universidade Nova de Lisboa, 2829516 Monte de Caparica, Portugal 5 b Instituto Superior de Ciencias da Saude Egas Moniz, Centro de Investigação Interdisciplinar Egas Moniz, P-2829511 Monte de Caparica, Caparica, Portugal 6 7 article info a b s t r a c t 1 9 10 Self-association constants for the flavylium cations of the six most common anthocyanidin 3-glucosides 20 11 were determined by circular dichroism (CD) and UV–Vis spectroscopy. -

Electrochemical and Antioxidant Properties of Anthocyanins and Anthocyanidins
CROATICA CHEMICA ACTA CCACAA 80 (1) 29¿34 (2007) ISSN-0011-1643 CCA-3135 Original Scientific Paper Electrochemical and Antioxidant Properties of Anthocyanins and Anthocyanidins Andréia A. de Lima, Eliana M. Sussuchi, and Wagner F. De Giovani* Departamento de Química, Faculdade de Filosofia, Ciências e Letras de Ribeirão Preto, Universidade de São Paulo, Av. Bandeirantes 3900, 14040-901, Ribeirão Preto – SP, Brazil RECEIVED JUNE 26, 2006; REVISED SEPTEMBER 29, 2006; ACCEPTED OCTOBER 27, 2006 Electrochemical properties of delphinidin, cyanidin, pelargonidin, kuromanin and callistephin were investigated by cyclic and differential pulse voltammetries at different pH values and also in methanol. On the basis of oxidation potentials, the order of antioxidant activity for antho- cyanidins is delphinidin > cyanidin > pelargonidin. Oxidation peaks for anthocyanins (kuro- manin and callistephin) are shifted to more positive potentials compared to anthocyanidins (delphinidin, cyanidin and pelargonidin). Oxidation peak currents are linearly dependent on the Keywords square root of the scan rate, which is typical of a diffusion controlled electrochemical process. anthocyanins Antioxidant activities of the compounds were evaluated using the 1,1-diphenyl-2-picrylhydra- anthocyanidins zyl (DPPH) radical-scavenging method and they are directly related to their redox potential antioxidants values. The order of the antioxidant activity is delphinidin > cyanidin > pelargonidin > kuro- electrochemical properties manin > callistephin. INTRODUCTION pharmacological properties that render them interesting potential cancer chemopreventive agents.9–11 Because Anthocyanins are one of the main classes of flavonoids anthocyanins are widely consumed, finding out addi- in red wines. They have been pointed out as the molecu- tional biological activities related to these compounds lar entities that are most likely responsible for what has would be of great interest. -

Callistephin Enhances the Protective Effects of Isoflurane on Microglial Injury Through Downregulation of Inflammation and Apoptosis
802 MOLECULAR MEDICINE REPORTS 20: 802-812, 2019 Callistephin enhances the protective effects of isoflurane on microglial injury through downregulation of inflammation and apoptosis LILI ZHAO, SHIBIAO CHEN, TIANYIN LIU, XIUHONG WANG, HAIJIN HUANG and WEICHENG LIU Department of Anesthesiology, The First Affiliated Hospital of Nanchang University, Nanchang, Jiangxi 330006, P.R. China Received June 18, 2018; Accepted March 15, 2019 DOI: 10.3892/mmr.2019.10282 Abstract. Microglia are the major immune cells in the central enhanced the effects of isoflurane. Callistephin may therefore nervous system. Microglial activation can be beneficial or constitute a candidate drug agent that may target inflammatory detrimental depending on the stimuli and the physiopathological and growth regulatory signaling pathways, thus ameliorating environment. Microglial activation is involved in a variety certain aspects of neurodegenerative diseases. of neurodegenerative disorders. Different anesthetic agents have exhibited diverse effects on microglial activation and Introduction the engulfment process. The anthocyanin callistephin has been demonstrated to have antioxidant and anti‑inflammatory Microglial cells are the major immune cell in the central properties, and these were assessed in the present study, with a nervous system (CNS), responding against types of endog- focus on its effect on microglial activation. Mouse microglial enous and exogenous stimuli, including infection by bacteria, cells C8-4B were treated with 100 ng/µl lipopolysaccharide viruses, prions and β-amyloid plaques (1). Microglia are (LPS) and 1 ng/µl interferon-γ. Cells were subsequently treated activated upon exposure to different stimuli and, depending with 2% isoflurane, 100 µM callistephin or both. LPS promoted on the environmental context, this may be beneficial or detri- apoptosis in C8-B4 cells, and this was reduced following mental to the functionality and physiology of the CNS (2). -

UNITED STATES PATENT OFFICE 2,636,042 WATER-SOLUBLE HORMONE COMPOUNDS Ralph Salkin, Jackson Heights, N.Y., Assignor to S
Patented Apr. 21, 1953 2,636,042 UNITED STATES PATENT OFFICE 2,636,042 WATER-SOLUBLE HORMONE COMPOUNDS Ralph Salkin, Jackson Heights, N.Y., assignor to S. B. Penick and Company, New York, N. Y., a corporation of Delaware No Drawing. Application July 8, 1949 Serial No. 103,759 5 Claims. (C. 260-39.4) 1. 2 My invention relates to an improvement in the ether, and the sulfate is then Salted out of the manufacture of water-soluble compounds of the aqueous solution by the addition of a, caustic estrane series, and in particular it is concerned solution under cooling. The liberated hormone With an improvement in the synthesis of alkali sulfate is extracted into a suitable Solvent, for and alkaline-earth metal salts of the sulfates of 5 instance butanol, pyridine being preferred how the estranes. ever. The hormone sulfate solution is exhaus The estranes to which my invention applies are tively extracted with ether to remove the solvent. steroids having a free hydroxyl group in the The resultant semicrystalline product is recrys 3-position and a hydroxy or keto group in the tallized from a dilute monohydric alcohol. Or 17-position of the molecule, such as estrone, O Water to give the pure sterol.ester. equilin, equilenin, estradiol and similar com In order to get pure ester Salts, I have found pounds. it essential that the tertiary amine-sulfur trioxide These products which are commonly known as adduct be absolutely pure when being reacted With conjugated estrogens can be obtained from nat the hormones. Improved yields and more readily ural sources such as the urine of pregnant mares purifiable light colored granular products result, or of stallions. -

Properties of Chemically Oxidized Kininogens*
Vol. 50 No. 3/2003 753–763 QUARTERLY Properties of chemically oxidized kininogens*. Magdalena Nizio³ek, Marcin Kot, Krzysztof Pyka, Pawe³ Mak and Andrzej Kozik½ Faculty of Biotechnology, Jagiellonian University, Kraków, Poland Received: 30 May, 2003; revised: 01 August, 2003; accepted: 11 August, 2003 Key words: bradykinin, N-chlorosuccinimide, chloramine-T, kallidin, kallikrein, reactive oxygen species Kininogens are multifunctional proteins involved in a variety of regulatory pro- cesses including the kinin-formation cascade, blood coagulation, fibrynolysis, inhibi- tion of cysteine proteinases etc. A working hypothesis of this work was that the prop- erties of kininogens may be altered by oxidation of their methionine residues by reac- tive oxygen species that are released at the inflammatory foci during phagocytosis of pathogen particles by recruited neutrophil cells. Two methionine-specific oxidizing reagents, N-chlorosuccinimide (NCS) and chloramine-T (CT), were used to oxidize the high molecular mass (HK) and low molecular mass (LK) forms of human kininogen. A nearly complete conversion of methionine residues to methionine sulfoxide residues in the modified proteins was determined by amino acid analysis. Production of kinins from oxidized kininogens by plasma and tissue kallikreins was significantly lower (by at least 70%) than that from native kininogens. This quenching effect on kinin release could primarily be assigned to the modification of the critical Met-361 residue adja- cent to the internal kinin sequence in kininogen. However, virtually no kinin could be formed by human plasma kallikrein from NCS-modified HK. This observation sug- gests involvement of other structural effects detrimental for kinin production. In- deed, NCS-oxidized HK was unable to bind (pre)kallikrein, probably due to the modifi- cation of methionine and/or tryptophan residues at the region on the kininogen mole- cule responsible for the (pro)enzyme binding. -

(Piper Nigrum L.) Products Based on LC-MS/MS Analysis
molecules Article Nontargeted Metabolomics for Phenolic and Polyhydroxy Compounds Profile of Pepper (Piper nigrum L.) Products Based on LC-MS/MS Analysis Fenglin Gu 1,2,3,*, Guiping Wu 1,2,3, Yiming Fang 1,2,3 and Hongying Zhu 1,2,3,* 1 Spice and Beverage Research Institute, Chinese Academy of Tropical Agricultural Sciences, Wanning 571533, China; [email protected] (G.W.); [email protected] (Y.F.) 2 National Center of Important Tropical Crops Engineering and Technology Research, Wanning 571533, China 3 Key Laboratory of Genetic Resources Utilization of Spice and Beverage Crops, Ministry of Agriculture, Wanning 571533, China * Correspondence: [email protected] (F.G.); [email protected] (H.Z.); Tel.: +86-898-6255-3687 (F.G.); +86-898-6255-6090 (H.Z.); Fax: +86-898-6256-1083 (F.G. & H.Z.) Received: 16 July 2018; Accepted: 7 August 2018; Published: 9 August 2018 Abstract: In the present study, nontargeted metabolomics was used to screen the phenolic and polyhydroxy compounds in pepper products. A total of 186 phenolic and polyhydroxy compounds, including anthocyanins, proanthocyanidins, catechin derivatives, flavanones, flavones, flavonols, isoflavones and 3-O-p-coumaroyl quinic acid O-hexoside, quinic acid (polyhydroxy compounds), etc. For the selected 50 types of phenolic compound, except malvidin 3,5-diglucoside (malvin), 0 L-epicatechin and 4 -hydroxy-5,7-dimethoxyflavanone, other compound contents were present in high contents in freeze-dried pepper berries, and pinocembrin was relatively abundant in two kinds of pepper products. The score plots of principal component analysis indicated that the pepper samples can be classified into four groups on the basis of the type pepper processing. -

Labeling and Synthesis of Estrogens and Their Metabolites
Labeling and Synthesis of Estrogens and Their Metabolites Paula Kiuru University of Helsinki Faculty of Science Department of Chemistry Laboratory of Organic Chemistry P.O. Box 55, 00014 University of Helsinki, Finland ACADEMIC DISSERTATION To be presented with the permission of the Faculty of Science of the University of Helsinki, for public criticism in Auditorium A110 of the Department of Chemistry, A. I. Virtasen Aukio 1, Helsinki, on June 18th, 2005 at 12 o'clock noon Helsinki 2005 ISBN 952-91-8812-9 (paperback) ISBN 952-10-2507-7 (PDF) Helsinki 2005 Valopaino Oy. 1 ABSTRACT 3 ACKNOWLEDGMENTS 4 LIST OF ORIGINAL PUBLICATIONS 5 LIST OF ABBREVIATIONS 6 1. INTRODUCTION 7 1.1 Nomenclature of estrogens 8 1.2 Estrogen biosynthesis 10 1.3 Estrogen metabolism and cancer 10 1.3.1 Estrogen metabolism 11 1.3.2 Ratio of 2-hydroxylation and 16α-hydroxylation 12 1.3.3 4-Hydroxyestrogens and cancer 12 1.3.4 2-Methoxyestradiol 13 1.4 Structural and quantitative analysis of estrogens 13 1.4.1 Structural elucidation 13 1.4.2 Analytical techniques 15 1.4.2.1 GC/MS 16 1.4.2.2 LC/MS 17 1.4.2.3 Immunoassays 18 1.4.3 Deuterium labeled internal standards for GC/MS and LC/MS 19 1.4.4 Isotopic purity 20 1.5 Labeling of estrogens with isotopes of hydrogen 20 1.5.1 Deuterium-labeling 21 1.5.1.1 Mineral acid catalysts 21 1.5.1.2 CF3COOD as deuterating reagent 22 1.5.1.3 Base-catalyzed deuterations 24 1.5.1.4 Transition metal-catalyzed deuterations 25 1.5.1.5 Deuteration without catalyst 27 1.5.1.6 Halogen-deuterium exchange 27 1.5.1.7 Multistep labelings 28 1.5.1.8 Summary of deuterations 30 1.5.2 Enhancement of deuteration 30 1.5.2.1 Microwave irradiation 30 1.5.2.2 Ultrasound 31 1.5.3 Tritium labeling 32 1.6 Deuteration estrogen fatty acid esters 34 1.7 Synthesis of 2-methoxyestradiol 35 1.7.1 Halogenation 35 1.7.2 Nitration of estrogens 37 1.7.3 Formylation 38 1.7.4 Fries rearrangement 39 1.7.5 Other syntheses of 2-methoxyestradiol 39 1.7.6 Synthesis of 4-methoxyestrone 40 1.8 Synthesis of 2- and 4-hydroxyestrogens 41 2. -

Sized Neuropeptides
M ETHODS IN MOLECULAR BIOLOGY™ Series Editor John M. Walker School of Life Sciences University of Hertfordshire Hatfield, Hertfordshire, AL10 9AB, UK For further volumes: http://www.springer.com/series/7651 Neuropeptides Methods and Protocols Edited by Adalberto Merighi Dipartimento di Morfofisiologia Veterinaria, Università degli Studi di Torino, Grugliasco, TO, Italy; Istituto Nazionale di Neuroscienze (INN), Università degli Studi di Torino, Grugliasco, TO, Italy Editor Adalberto Merighi Dipartimento di Morfofisiologia Veterinaria Università degli Studi di Torino and Istituto Nazionale di Neuroscienze (INN) Università degli Studi di Torino Grugliasco, TO, Italy [email protected] Please note that additional material for this book can be downloaded from http://extras.springer.com ISSN 1064-3745 e-ISSN 1940-6029 ISBN 978-1-61779-309-7 e-ISBN 978-1-61779-310-3 DOI 10.1007/978-1-61779-310-3 Springer New York Dordrecht Heidelberg London Library of Congress Control Number: 2011936011 © Springer Science+Business Media, LLC 2011 All rights reserved. This work may not be translated or copied in whole or in part without the written permission of the publisher (Humana Press, c/o Springer Science+Business Media, LLC, 233 Spring Street, New York, NY 10013, USA), except for brief excerpts in connection with reviews or scholarly analysis. Use in connection with any form of information storage and retrieval, electronic adaptation, computer software, or by similar or dissimilar methodology now known or hereafter developed is forbidden. The use in this publication of trade names, trademarks, service marks, and similar terms, even if they are not identified as such, is not to be taken as an expression of opinion as to whether or not they are subject to proprietary rights. -

REVIEW Steroid Sulfatase Inhibitors for Estrogen
99 REVIEW Steroid sulfatase inhibitors for estrogen- and androgen-dependent cancers Atul Purohit and Paul A Foster1 Oncology Drug Discovery Group, Section of Investigative Medicine, Imperial College London, Hammersmith Hospital, London W12 0NN, UK 1School of Clinical and Experimental Medicine, Centre for Endocrinology, Diabetes and Metabolism, University of Birmingham, Birmingham B15 2TT, UK (Correspondence should be addressed to P A Foster; Email: [email protected]) Abstract Estrogens and androgens are instrumental in the maturation of in vivo and where we currently stand in regards to clinical trials many hormone-dependent cancers. Consequently,the enzymes for these drugs. STS inhibitors are likely to play an important involved in their synthesis are cancer therapy targets. One such future role in the treatment of hormone-dependent cancers. enzyme, steroid sulfatase (STS), hydrolyses estrone sulfate, Novel in vivo models have been developed that allow pre-clinical and dehydroepiandrosterone sulfate to estrone and dehydroe- testing of inhibitors and the identification of lead clinical piandrosterone respectively. These are the precursors to the candidates. Phase I/II clinical trials in postmenopausal women formation of biologically active estradiol and androstenediol. with breast cancer have been completed and other trials in This review focuses on three aspects of STS inhibitors: patients with hormone-dependent prostate and endometrial 1) chemical development, 2) biological activity, and 3) clinical cancer are currently active. Potent STS inhibitors should trials. The aim is to discuss the importance of estrogens and become therapeutically valuable in hormone-dependent androgens in many cancers, the developmental history of STS cancers and other non-oncological conditions. -

University Microfilms, Inc., Ann Arbor, Michigan ADRENOCORTICAL STEROID PROFILE IN
This dissertation has been Mic 61-2820 microfilmed exactly as received BESCH, Paige Keith. ADRENOCORTICAL STEROID PROFILE IN THE HYPERTENSIVE DOG. The Ohio State University, Ph.D., 1961 Chemistry, biological University Microfilms, Inc., Ann Arbor, Michigan ADRENOCORTICAL STEROID PROFILE IN THE HYPERTENSIVE DOG DISSERTATION Presented in Partial Fulfillment of the Requirements for the Degree Doctor of Philosophy in the Graduate School of the Ohio State University By Paige Keith Besch, B. S., M. S. The Ohio State University 1961 Approved by Katharine A. Brownell Department of Physiology DEDICATION This work is dedicated to my wife, Dr. Norma F. Besch. After having completed her graduate training, she was once again subjected to almost social isolation by the number of hours I spent away from home. It is with sincerest appreciation for her continual encouragement that I dedi cate this to her. ACKNOWLEDGMENTS I wish to acknowledge the assistance and encourage ment of my Professor, Doctor Katharine A. Brownell. Equally important to the development of this project are the experience and information obtained through the association with Doctor Frank A. Hartman, who over the years has, along with Doctor Brownell, devoted his life to the development of many of the techniques used in this study. It is also with extreme sincerity that I wish to ac knowledge the assistance of Mr. David J. Watson. He has never complained when asked to work long hours at night or weekends. Our association has been a fruitful one. I also wish to acknowledge the encouragement of my former Professor, employer and good friend, Doctor Joseph W. -

Characterisation of Bioactive Compounds in Berries from Plants Grown Under Innovative Photovoltaic Greenhouses
Journal of Berry Research 8 (2018) 55–69 55 DOI:10.3233/JBR-170258 IOS Press Characterisation of bioactive compounds in berries from plants grown under innovative photovoltaic greenhouses Federica Blandoa,∗, Carmela Gerardia, Massimiliano Rennab,c, Sergio Castellanod and Francesco Seriob aInstitute of Sciences of Food Production (ISPA), CNR, Lecce, Italy bInstitute of Sciences of Food Production (ISPA), CNR, Bari, Italy cDepartment of Agricultural and Environmental Science, University of Bari Aldo Moro, Bari, Italy dDepartment of Science of Agriculture, Food and Environment, (SAFE) University of Foggia, Foggia, Italy Received 2 July 2017; accepted 1 November 2017 Abstract. BACKGROUND: Bioactive compounds, mainly polyphenols, present in berries, are thought to be responsible for the health benefits of these fruit. Therefore, it is worthwhile to define the optimal environmental conditions to maximise their polyphenol content. OBJECTIVE: With the aim to define the optimal conditions for berry cultivation in an innovative environment, red rasp- berry, wild strawberry and blackberry plants were grown in a traditional greenhouse in comparison with two photovoltaic greenhouses with different shading area. METHODS: Hydroalcoholic extracts of ripe berries were evaluated by HPLC analysis, for their anthocyanins, organic acids and sugar contents. Moreover, phenolic content (by the Folin-Ciocalteu assay) and antioxidant activity (by the Trolox equivalent antioxidant capacity-TEAC assay) were assayed on the same berry extracts. RESULTS: Total anthocyanins, -

First-Pass Metabolism of Polyphenols from Selected Berries: a High-Throughput Bioanalytical Approach
antioxidants Article First-Pass Metabolism of Polyphenols from Selected Berries: A High-Throughput Bioanalytical Approach Francisco J. Olivas-Aguirre 1,*, Sandra Mendoza 2, Emilio Alvarez-Parrilla 3 , Gustavo A. Gonzalez-Aguilar 4 , Monica A. Villegas-Ochoa 4 , Jael T.J. Quintero-Vargas 1 and Abraham Wall-Medrano 3,* 1 Departamento de Ciencias de la Salud, Universidad de Sonora (Campus Cajeme), Blvd Bordo Nuevo s/n, Ejido Providencia, Cd, Obregón 85199, Mexico; [email protected] 2 Departamento de Investigación y Posgrado en Alimentos (PROPAC), Facultad de Química, Universidad Autónoma de Querétaro, Cerro de las Campanas s/n, Santiago de Querétaro 76010, Mexico; [email protected] 3 Instituto de Ciencias Biomédicas, Universidad Autónoma de Ciudad Juárez, Anillo Envolvente del PRONAF y Estocolmo s/n, Ciudad Juárez 32310, Mexico; [email protected] 4 Centro de Investigación en Alimentación y Desarrollo, A.C. (CIAD), Carretera a Ejido La Victoria, Km. 0.6, Hermosillo 83304, Mexico; [email protected] (G.A.G.-A.); [email protected] (M.A.V.-O.) * Correspondence: [email protected] (F.J.O.-A.); [email protected] (A.W.-M.) Received: 29 March 2020; Accepted: 11 April 2020; Published: 13 April 2020 Abstract: Small berries are rich in polyphenols whose first-pass metabolism may alter their ultimate physiological effects. The antioxidant capacity and polyphenol profile of three freeze-dried berries (blackberry, raspberry, Red Globe grape) were measured and their apparent permeability (Papp) and first-pass biotransformation were tracked with an ex vivo bioanalytical system [everted gut sac (rat) + three detection methods: spectrophotometry, HPLC-ESI-QTOF-MS, differential pulse voltammetry (DPV)].