DRAFT WRITTEN FINDINGS of the WASHINGTON STATE NOXIOUS WEED CONTROL BOARD Proposed Class a Noxious Weed Listing
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Germination, Vegetative and Flowering Behavior of Balsam (Impatiens
International Journal of Environment, Agriculture and Biotechnology (IJEAB) Vol-3, Issue -4, Jul-Aug- 2018 http://dx.doi.org/10.22161/ijeab/3.4.38 ISSN: 2456-1878 Germination, vegetative and flowering behavior of Balsam (Impatiens balsamina L.) in response to natural photoperiods Muhammad Aslam Baloch1, Tanveer Fatima Miano*1, Niaz Ahmed Wahocho1, Naheed Akhtar Talpur2, Abdul Qadir Gola1 1Department of Horticulture, Sindh Agriculture University Tandojam, Pakistan 2Department of Soil Science, Sindh Agriculture University Tandojam, Pakistan Corresponding Author: Dr. Tanveer Fatima Miano*, Associate Professor Corresponding Author Email: [email protected] Abstract— A lack of application of photoperiod and light content (46.79 SPAD) was recorded from NP4= 9 hrs intensity to manipulate the growth of current spring (8:00 am-5:00 pm) as compared to seed germination annuals has, in part, been due to the lack of information (63.88%), germination index (0.66 gi) plant height (14.92 identifying the photoperiodic and light intensities cm), leaves plant-1 (48.16), days to 1st flower (45.96), requirements of various species. Present pot experiment flowers plant-1 (5.00), days to flower persistence (11.16), was carried out at Horticulture Garden, Department of weight of single flower (0.62 g), chlorophyll content (36.46 Horticulture, Sindh Agriculture University Tandojam, SPAD) was recorded from NP1= Control (Normal day during spring 2017, which was laid out in a three length). replicated Complete Randomized Design (CRD). Two Keywords— flowering behaviour, natural photoperiods, varieties of balsam (V1= Tom Thumb, V2 = Double Complete Randomized Design. Camcellia) were studied under NP1= Control (Normal day length), NP2=3 hrs (8:00 am- 11:00 am), NP3= 6 hrs (8:00 I. -

Cytomorphological Diversity in Some Species of Impatiens Linn. (Balsaminaceae) from Western Himalayas (India)
© 2010 The Japan Mendel Society Cytologia 75(4): 379–387, 2010 Cytomorphological Diversity in Some Species of Impatiens Linn. (Balsaminaceae) from Western Himalayas (India) Syed Mudassir Jeelani*, Savita Rani, Sanjeev Kumar, Raghbir Chand Gupta and Santosh Kumari Department of Botany, Punjabi University, Patiala 147 002, India Received May 28, 2010; accepted August 28, 2010 Summary The genus Impatiens Linn. belongs to the family Balsaminaceae and includes mostly wild as well as commonly cultivated ornamental plants. Nearly 91% of Indian species of Impatiens are reported to be endemic. To generate basic information on genetic diversity required for the improvement of germplasm, the present study has been carried out from the different selected parts of Western Himalayas such as Kashmir (J&K) and the Kangra and Sirmaur districts (H.P). During this study, 23 accessions belonging to 9 species of the genus Impatiens have been cytomorphologi- cally observed. The species being cytologically worked out for the first time on a worldwide basis include 2 species as I.laxiflora (nϭ7, 8) and I. reidii (nϭ7). Six aneuploid cytotypes have been reported for the first time for the species I. arguta (nϭ7), I. bicornuta (nϭ7), I. brachycentra (nϭ8), I. glandulifera (nϭ6), I. scabrida (nϭ6) and I. sulcata (nϭ8) on a worldwide basis. The meiotic course in most of these accessions has been observed to be normal except for some of the accessions of I. brachycentra, I. glandulifera, I. scabrida and I. sulcata marked with abnormal meiosis. Out of 4 species (6 accessions) marked with cytomixis, in 2 accessions, one for each of I. scabrida and I. -

Impatiens Glandulifera (Himalayan Balsam) Chloroplast Genome Sequence As a Promising Target for Populations Studies
Impatiens glandulifera (Himalayan balsam) chloroplast genome sequence as a promising target for populations studies Giovanni Cafa1, Riccardo Baroncelli2, Carol A. Ellison1 and Daisuke Kurose1 1 CABI Europe, Egham, Surrey, UK 2 University of Salamanca, Instituto Hispano-Luso de Investigaciones Agrarias (CIALE), Villamayor (Salamanca), Spain ABSTRACT Background: Himalayan balsam Impatiens glandulifera Royle (Balsaminaceae) is a highly invasive annual species native of the Himalayas. Biocontrol of the plant using the rust fungus Puccinia komarovii var. glanduliferae is currently being implemented, but issues have arisen with matching UK weed genotypes with compatible strains of the pathogen. To support successful biocontrol, a better understanding of the host weed population, including potential sources of introductions, of Himalayan balsam is required. Methods: In this molecular study, two new complete chloroplast (cp) genomes of I. glandulifera were obtained with low coverage whole genome sequencing (genome skimming). A 125-year-old herbarium specimen (HB92) collected from the native range was sequenced and assembled and compared with a 2-year-old specimen from UK field plants (HB10). Results: The complete cp genomes were double-stranded molecules of 152,260 bp (HB92) and 152,203 bp (HB10) in length and showed 97 variable sites: 27 intragenic and 70 intergenic. The two genomes were aligned and mapped with two closely related genomes used as references. Genome skimming generates complete organellar genomes with limited technical and financial efforts and produces large datasets compared to multi-locus sequence typing. This study demonstrates the 26 July 2019 Submitted suitability of genome skimming for generating complete cp genomes of historic Accepted 12 February 2020 Published 24 March 2020 herbarium material. -

Impatiens Glandulifera
NOBANIS – Invasive Alien Species Fact Sheet Impatiens glandulifera Author of this fact sheet: Harry Helmisaari, SYKE (Finnish Environment Institute), P.O. Box 140, FIN-00251 Helsinki, Finland, Phone + 358 20 490 2748, E-mail: [email protected] Bibliographical reference – how to cite this fact sheet: Helmisaari, H. (2010): NOBANIS – Invasive Alien Species Fact Sheet – Impatiens glandulifera. – From: Online Database of the European Network on Invasive Alien Species – NOBANIS www.nobanis.org, Date of access x/x/201x. Species description Scientific name: Impatiens glandulifera Royle (Balsaminaceae). Synonyms: Impatiens roylei Walpers. Common names: Himalayan balsam, Indian balsam, Policeman's Helmet (GB), Drüsiges Springkraut, Indisches Springkraut (DE), kæmpe-balsamin (DK), verev lemmalts (EE), jättipalsami (FI), risalísa (IS), bitinė sprigė (LT), puķu sprigane (LV), Reuzenbalsemien (NL), kjempespringfrø (NO), Niecierpek gruczolowaty, Niecierpek himalajski (PL), недотрога железконосная (RU), jättebalsamin (SE). Fig. 1 and 2. Impatiens glandulifera in an Alnus stand in Helsinki, Finland, and close-up of the seed capsules, photos by Terhi Ryttäri and Harry Helmisaari. Fig. 3 and 4. White and red flowers of Impatiens glandulifera, photos by Harry Helmisaari. Species identification Impatiens glandulifera is a tall annual with a smooth, usually hollow and jointed stem, which is easily broken (figs. 1-4). The stem can reach a height of 3 m and its diameter can be up to several centimetres. The leaves are opposite or in whorls of 3, glabrous, lanceolate to elliptical, 5-18 cm long and 2.5-7 cm wide. The inflorescences are racemes of 2-14 flowers that are 25-40 mm long. Flowers are zygomorphic, their lowest sepal forming a sac that ends in a straight spur. -

(Balsaminaceae) Using Chloroplast Atpb-Rbcl Spacer Sequences
Systematic Botany (2006), 31(1): pp. 171–180 ᭧ Copyright 2006 by the American Society of Plant Taxonomists Phylogenetics of Impatiens and Hydrocera (Balsaminaceae) Using Chloroplast atpB-rbcL Spacer Sequences STEVEN JANSSENS,1,4 KOEN GEUTEN,1 YONG-MING YUAN,2 YI SONG,2 PHILIPPE KU¨ PFER,2 and ERIK SMETS1,3 1Laboratory of Plant Systematics, Institute of Botany and Microbiology, Kasteelpark Arenberg 31, B-3001 Leuven, Belgium; 2Institut de Botanique, Universite´ de Neuchaˆtel, Neuchaˆtel, Switzerland; 3Nationaal Herbarium Nederland, Universiteit Leiden Branch, PO Box 9514, 2300 RA Leiden, The Netherlands 4Author for correspondence ([email protected]) ABSTRACT. Balsaminaceae are a morphologically diverse family with ca. 1,000 representatives that are mainly distributed in the Old World tropics and subtropics. To understand the relationships of its members, we obtained chloroplast atpB-rbcL sequences from 86 species of Balsaminaceae and five outgroups. Phylogenetic reconstructions using parsimony and Bayesian approaches provide a well-resolved phylogeny in which the sister group relationship between Impatiens and Hydrocera is confirmed. The overall topology of Impatiens is strongly supported and is geographically structured. Impatiens likely origi- nated in South China from which it colonized the adjacent regions and afterwards dispersed into North America, Africa, India, the Southeast Asian peninsula, and the Himalayan region. Balsaminaceae are annual or perennial herbs with in the intrageneric relationships of Impatiens. The most flowers that exhibit a remarkable diversity. The family recent molecular intrageneric study on Balsaminaceae consists of more than 1,000 species (Grey-Wilson utilized Internal Transcribed Spacer (ITS) sequences 1980a; Clifton 2000), but only two genera are recog- and provided new phylogenetic insights in Impatiens nized. -
Summary Data
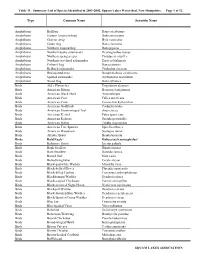
Table 11. Summary List of Species Identified in 2001-2002, Squam Lakes Watershed, New Hampshire. Page 1 of 12 Type Common Name Scientific Name Amphibians Bullfrog Rana catesbeiana Amphibians Eastern American toad Bufo americanus Amphibians Gray treefrog Hyla versicolor Amphibians Green frog Rana clamitans Amphibians Northern leopard frog Rana pipiens Amphibians Northern dusky salamander Desmognathus fuscus Amphibians Northern spring peeper Pseudacris crucifer Amphibians Northern two-lined salamander Eurycea bislineata Amphibians Pickerel frog Rana palustris Amphibians Redback salamander Plethodon cinereus Amphibians Red-spotted newt Notophthalmus viridescens Amphibians Spotted salamander Ambystoma maculatum Amphibians Wood frog Rana sylvatica Birds Alder Flycatcher Empidonax alnorum Birds American Bittern Botaurus lentiginosus Birds American Black Duck Anas rubripes Birds American Coot Fulica americana Birds American Crow Corvus brachyrhynchos Birds American Goldfinch Carduelis tristis Birds American Green-winged Teal Anas crecca Birds American Kestrel Falco sparverius Birds American Redstart Setophaga ruticilla Birds American Robin Turdus migratorius Birds American Tree Sparrow Spizella arborea Birds American Woodcock Scolopax minor Birds Atlantic Brant Branta bernicla Birds Bald Eagle* Haliaeetus leucocephalus* Birds Baltimore Oriole Icterus galbula Birds Bank Swallow Riparia riparia Birds Barn Swallow Hirundo rustica Birds Barred Owl Strix varia Birds Belted Kingfisher Ceryle alcyon Birds Black-and-white Warbler Mniotilta varia Birds -

Impatiens Walleriana (Balsaminaceae)
Phytotaxa 3: 62–62 (2010) ISSN 1179-3155 (print edition) www.mapress.com/phytotaxa/ Correspondence PHYTOTAXA Copyright © 2010 • Magnolia Press ISSN 1179-3163 (online edition) Typification of ornamental plants 4: Impatiens walleriana (Balsaminaceae) MAARTEN J. M. CHRISTENHUSZ Department of Botany, The Natural History Museum, Cromwell Road, London SW7 5BD, United Kingdom. Impatiens walleriana Hook.f. in Oliver (1868: 302). Protologue: “Mozambique District, Moramballa, 2000 ft., on stones in streams”. Syntypes: J. Kirk s.n. (K!); H. Waller s.n. (K!). Lectotype (designated here): Mozambique, Moramballa, 0-3000 ft., Zambesi Expedition, H. Waller s.n. (K!-000419538). Notes: The name was published as ‘Walleriana’, which according to the ICBN (McNeill et al., 2006) is not a correctable error. The species is sometimes erroneously cited as ‘I. wallerana’. The other syntype, J. Kirk s.n. (K!), is also the holotype of Impatiens sultanii Hooker (1882: t. 6643), a synonym. The well-known bedding plant ‘busy lizzie’, Impatiens walleriana (Balsaminaceae), is a species occurring naturally in East Africa, where it can be found locally abundant in Kenya, Tanzania and Mozambique. It is frequently associated with wet or humid habitats and can thus be found near streams, waterfalls and in gorges or in the understorey of wet forests. Elsewhere in the tropics and subtropics the species can commonly be found naturalised along roads, in secondary forests and other disturbed habitats, where it can form dominant stands, competing out other species (Richardson et al. 2000, Tabak & Wettenberg 2008). Impatiens walleriana was first discovered during one of Dr David Livingstone’s expeditions up the Zambezi River, where he travelled with Dr John Kirk and Ref. -

Phylogenetic Relationships in the Order Ericales S.L.: Analyses of Molecular Data from Five Genes from the Plastid and Mitochondrial Genomes1
American Journal of Botany 89(4): 677±687. 2002. PHYLOGENETIC RELATIONSHIPS IN THE ORDER ERICALES S.L.: ANALYSES OF MOLECULAR DATA FROM FIVE GENES FROM THE PLASTID AND MITOCHONDRIAL GENOMES1 ARNE A. ANDERBERG,2,5 CATARINA RYDIN,3 AND MARI KAÈ LLERSJOÈ 4 2Department of Phanerogamic Botany, Swedish Museum of Natural History, P.O. Box 50007, SE-104 05 Stockholm, Sweden; 3Department of Systematic Botany, University of Stockholm, SE-106 91 Stockholm, Sweden; and 4Laboratory for Molecular Systematics, Swedish Museum of Natural History, P.O. Box 50007, SE-104 05 Stockholm, Sweden Phylogenetic interrelationships in the enlarged order Ericales were investigated by jackknife analysis of a combination of DNA sequences from the plastid genes rbcL, ndhF, atpB, and the mitochondrial genes atp1 and matR. Several well-supported groups were identi®ed, but neither a combination of all gene sequences nor any one alone fully resolved the relationships between all major clades in Ericales. All investigated families except Theaceae were found to be monophyletic. Four families, Marcgraviaceae, Balsaminaceae, Pellicieraceae, and Tetrameristaceae form a monophyletic group that is the sister of the remaining families. On the next higher level, Fouquieriaceae and Polemoniaceae form a clade that is sister to the majority of families that form a group with eight supported clades between which the interrelationships are unresolved: Theaceae-Ternstroemioideae with Ficalhoa, Sladenia, and Pentaphylacaceae; Theaceae-Theoideae; Ebenaceae and Lissocarpaceae; Symplocaceae; Maesaceae, Theophrastaceae, Primulaceae, and Myrsinaceae; Styr- acaceae and Diapensiaceae; Lecythidaceae and Sapotaceae; Actinidiaceae, Roridulaceae, Sarraceniaceae, Clethraceae, Cyrillaceae, and Ericaceae. Key words: atpB; atp1; cladistics; DNA; Ericales; jackknife; matR; ndhF; phylogeny; rbcL. Understanding of phylogenetic relationships among angio- was available for them at the time, viz. -

How Does Genome Size Affect the Evolution of Pollen Tube Growth Rate, a Haploid Performance Trait?
Manuscript bioRxiv preprint doi: https://doi.org/10.1101/462663; this version postedClick April here18, 2019. to The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv aaccess/download;Manuscript;PTGR.genome.evolution.15April20 license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. 1 Effects of genome size on pollen performance 2 3 4 5 How does genome size affect the evolution of pollen tube growth rate, a haploid 6 performance trait? 7 8 9 10 11 John B. Reese1,2 and Joseph H. Williams2 12 Department of Ecology and Evolutionary Biology, University of Tennessee, Knoxville, TN 13 37996, U.S.A. 14 15 16 17 1Author for correspondence: 18 John B. Reese 19 Tel: 865 974 9371 20 Email: [email protected] 21 1 bioRxiv preprint doi: https://doi.org/10.1101/462663; this version posted April 18, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. 22 ABSTRACT 23 Premise of the Study – Male gametophytes of most seed plants deliver sperm to eggs via a 24 pollen tube. Pollen tube growth rates (PTGRs) of angiosperms are exceptionally rapid, a pattern 25 attributed to more effective haploid selection under stronger pollen competition. Paradoxically, 26 whole genome duplication (WGD) has been common in angiosperms but rare in gymnosperms. -

Comparative Genomics of the Balsaminaceae Sister Genera Hydrocera Triflora and Impatiens Pinfanensis
International Journal of Molecular Sciences Article Comparative Genomics of the Balsaminaceae Sister Genera Hydrocera triflora and Impatiens pinfanensis Zhi-Zhong Li 1,2,†, Josphat K. Saina 1,2,3,†, Andrew W. Gichira 1,2,3, Cornelius M. Kyalo 1,2,3, Qing-Feng Wang 1,3,* and Jin-Ming Chen 1,3,* ID 1 Key Laboratory of Aquatic Botany and Watershed Ecology, Wuhan Botanical Garden, Chinese Academy of Sciences, Wuhan 430074, China; [email protected] (Z.-Z.L.); [email protected] (J.K.S.); [email protected] (A.W.G.); [email protected] (C.M.K.) 2 University of Chinese Academy of Sciences, Beijing 100049, China 3 Sino-African Joint Research Center, Chinese Academy of Sciences, Wuhan 430074, China * Correspondence: [email protected] (Q.-F.W.); [email protected] (J.-M.C.); Tel.: +86-27-8751-0526 (Q.-F.W.); +86-27-8761-7212 (J.-M.C.) † These authors contributed equally to this work. Received: 21 December 2017; Accepted: 15 January 2018; Published: 22 January 2018 Abstract: The family Balsaminaceae, which consists of the economically important genus Impatiens and the monotypic genus Hydrocera, lacks a reported or published complete chloroplast genome sequence. Therefore, chloroplast genome sequences of the two sister genera are significant to give insight into the phylogenetic position and understanding the evolution of the Balsaminaceae family among the Ericales. In this study, complete chloroplast (cp) genomes of Impatiens pinfanensis and Hydrocera triflora were characterized and assembled using a high-throughput sequencing method. The complete cp genomes were found to possess the typical quadripartite structure of land plants chloroplast genomes with double-stranded molecules of 154,189 bp (Impatiens pinfanensis) and 152,238 bp (Hydrocera triflora) in length. -

How Does Genome Size Affect the Evolution of Pollen Tube Growth Rate, a Haploid Performance Trait?
bioRxiv preprint doi: https://doi.org/10.1101/462663; this version posted November 5, 2018. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. 1 Effects of genome size on pollen performance 2 3 4 5 6 How does genome size affect the evolution of pollen tube growth rate, a haploid 7 performance trait? 8 9 10 11 12 John B. Reese1,2 and Joseph H. Williams1 13 Department of Ecology and Evolutionary Biology, University of Tennessee, Knoxville, TN 14 37996, U.S.A. 15 16 17 18 1Author for correspondence: 19 John B. Reese 20 Tel: 865 974 9371 21 Email: [email protected] 22 23 24 1 bioRxiv preprint doi: https://doi.org/10.1101/462663; this version posted November 5, 2018. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. 25 ABSTRACT 26 Premise of the Study - Male gametophytes of seed plants deliver sperm to eggs via a pollen 27 tube. Pollen tube growth rate (PTGR) may evolve rapidly due to pollen competition and haploid 28 selection, but many angiosperms are currently polyploid and all have polyploid histories. 29 Polyploidy should initially accelerate PTGR via “genotypic effects” of increased gene dosage 30 and heterozygosity on metabolic rates, but “nucleotypic effects” of genome size on cell size 31 should reduce PTGR. -

583–584 Angiosperms 583 *Eudicots and Ceratophyllales
583 583 > 583–584 Angiosperms These schedules are extensively revised, having been prepared with little reference to earlier editions. 583 *Eudicots and Ceratophyllales Subdivisions are added for eudicots and Ceratophyllales together, for eudicots alone Class here angiosperms (flowering plants), core eudicots For monocots, basal angiosperms, Chloranthales, magnoliids, see 584 See Manual at 583–585 vs. 600; also at 583–584; also at 583 vs. 582.13 .176 98 Mangrove swamp ecology Number built according to instructions under 583–588 Class here comprehensive works on mangroves For mangroves of a specific order or family, see the order or family, e.g., mangroves of family Combretaceae 583.73 .2 *Ceratophyllales Class here Ceratophyllaceae Class here hornworts > 583.3–583.9 Eudicots Class comprehensive works in 583 .3 *Ranunculales, Sabiaceae, Proteales, Trochodendrales, Buxales .34 *Ranunculales Including Berberidaceae, Eupteleaceae, Menispermaceae, Ranunculaceae Including aconites, anemones, barberries, buttercups, Christmas roses, clematises, columbines, delphiniums, hellebores, larkspurs, lesser celandine, mandrake, mayapple, mayflower, monkshoods, moonseeds, wolfsbanes For Fumariaceae, Papaveraceae, Pteridophyllaceae, see 583.35 See also 583.9593 for mandrakes of family Solanaceae .35 *Fumariaceae, Papaveraceae, Pteridophyllaceae Including bleeding hearts, bloodroot, celandines, Dutchman’s breeches, fumitories, poppies See also 583.34 for lesser celandine .37 *Sabiaceae * *Add as instructed under 583–588 1 583 Dewey Decimal Classification