Variation and Macroevolution in Leaf Functional Traits in the Hawaiian Silversword Alliance (Asteraceae)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

"National List of Vascular Plant Species That Occur in Wetlands: 1996 National Summary."
Intro 1996 National List of Vascular Plant Species That Occur in Wetlands The Fish and Wildlife Service has prepared a National List of Vascular Plant Species That Occur in Wetlands: 1996 National Summary (1996 National List). The 1996 National List is a draft revision of the National List of Plant Species That Occur in Wetlands: 1988 National Summary (Reed 1988) (1988 National List). The 1996 National List is provided to encourage additional public review and comments on the draft regional wetland indicator assignments. The 1996 National List reflects a significant amount of new information that has become available since 1988 on the wetland affinity of vascular plants. This new information has resulted from the extensive use of the 1988 National List in the field by individuals involved in wetland and other resource inventories, wetland identification and delineation, and wetland research. Interim Regional Interagency Review Panel (Regional Panel) changes in indicator status as well as additions and deletions to the 1988 National List were documented in Regional supplements. The National List was originally developed as an appendix to the Classification of Wetlands and Deepwater Habitats of the United States (Cowardin et al.1979) to aid in the consistent application of this classification system for wetlands in the field.. The 1996 National List also was developed to aid in determining the presence of hydrophytic vegetation in the Clean Water Act Section 404 wetland regulatory program and in the implementation of the swampbuster provisions of the Food Security Act. While not required by law or regulation, the Fish and Wildlife Service is making the 1996 National List available for review and comment. -

THE JEPSON GLOBE a Newsletter from the Friends of the Jepson Herbarium
THE JEPSON GLOBE A Newsletter from the Friends of The Jepson Herbarium VOLUME 26 NUMBER 1, Spring 2016 Curator’s Column: Museomics The Jepson Manual: Vascular Reveals Secrets of the Dead Plants of California, Second By Bruce G. Baldwin Edition: Supplement III Over the last decade, herbaria By Bruce G. Baldwin have received well-deserved public- The latest set of revisions to The Jep- ity as treasure troves of undiscovered son Manual, second edition (TJM2) and biodiversity, with the recognition that the Jepson eFlora was released online most “new” species named in the last in December 2015. The rapid pace of half-century have long resided in col- discovery and description of vascular lections prior to their detection and plant taxa that are new-to-science for original description. The prospect also California and the rarity and endanger- has emerged for unlocking the secrets of ment of most of those new taxa have plants and other organisms that no lon- warranted prioritization of revisions ger share our planet as living organisms that incorporate such diversity — and and, sadly, reside only in collections. Map of California, split apart to show newly introduced, putatively aggressive Technological advances that now al- the Regions of the Jepson eFlora. invasives — so that detection of such low for DNA sequencing on a genomic Source: Jepson Flora Project. plants in the field and in collections scale also are well suited for studying Regional dichotomous keys now is not impeded. The continuing taxo- old, highly degraded specimens, as re- nomic reorganization of genera and, to cent reconstruction of the Neanderthal available for the Jepson eFlora some extent, families in order to reflect genome has shown. -

Spatial Models of Speciation 1.0Cm Modelos Espaciais De Especiação
UNIVERSIDADE ESTADUAL DE CAMPINAS INSTITUTO DE BIOLOGIA CAROLINA LEMES NASCIMENTO COSTA SPATIAL MODELS OF SPECIATION MODELOS ESPACIAIS DE ESPECIAÇÃO CAMPINAS 2019 CAROLINA LEMES NASCIMENTO COSTA SPATIAL MODELS OF SPECIATION MODELOS ESPACIAIS DE ESPECIAÇÃO Thesis presented to the Institute of Biology of the University of Campinas in partial fulfill- ment of the requirements for the degree of Doc- tor in Ecology Tese apresentada ao Instituto de Biologia da Universidade Estadual de Campinas como parte dos requisitos exigidos para a obtenção do título de Doutora em Ecologia Orientador: Marcus Aloizio Martinez de Aguiar ESTE ARQUIVO DIGITAL CORRESPONDE À VERSÃO FINAL DA TESE DEFENDIDA PELA ALUNA CAROLINA LEMES NASCIMENTO COSTA, E ORIENTADA PELO PROF DR. MAR- CUS ALOIZIO MARTINEZ DE AGUIAR. CAMPINAS 2019 Ficha catalográfica Universidade Estadual de Campinas Biblioteca do Instituto de Biologia Mara Janaina de Oliveira - CRB 8/6972 Costa, Carolina Lemes Nascimento, 1989- C823s CosSpatial models of speciation / Carolina Lemes Nascimento Costa. – Campinas, SP : [s.n.], 2019. CosOrientador: Marcus Aloizio Martinez de Aguiar. CosTese (doutorado) – Universidade Estadual de Campinas, Instituto de Biologia. Cos1. Especiação. 2. Radiação adaptativa (Evolução). 3. Modelos biológicos. 4. Padrão espacial. 5. Macroevolução. I. Aguiar, Marcus Aloizio Martinez de, 1960-. II. Universidade Estadual de Campinas. Instituto de Biologia. III. Título. Informações para Biblioteca Digital Título em outro idioma: Modelos espaciais de especiação Palavras-chave em inglês: Speciation Adaptive radiation (Evolution) Biological models Spatial pattern Macroevolution Área de concentração: Ecologia Titulação: Doutora em Ecologia Banca examinadora: Marcus Aloizio Martinez de Aguiar [Orientador] Mathias Mistretta Pires Sabrina Borges Lino Araujo Rodrigo André Caetano Gustavo Burin Ferreira Data de defesa: 25-02-2019 Programa de Pós-Graduação: Ecologia Powered by TCPDF (www.tcpdf.org) Comissão Examinadora: Prof. -

A Landscape-Based Assessment of Climate Change Vulnerability for All Native Hawaiian Plants
Technical Report HCSU-044 A LANDscape-bASED ASSESSMENT OF CLIMatE CHANGE VULNEraBILITY FOR ALL NatIVE HAWAIIAN PLANts Lucas Fortini1,2, Jonathan Price3, James Jacobi2, Adam Vorsino4, Jeff Burgett1,4, Kevin Brinck5, Fred Amidon4, Steve Miller4, Sam `Ohukani`ohi`a Gon III6, Gregory Koob7, and Eben Paxton2 1 Pacific Islands Climate Change Cooperative, Honolulu, HI 96813 2 U.S. Geological Survey, Pacific Island Ecosystems Research Center, Hawaii National Park, HI 96718 3 Department of Geography & Environmental Studies, University of Hawai‘i at Hilo, Hilo, HI 96720 4 U.S. Fish & Wildlife Service —Ecological Services, Division of Climate Change and Strategic Habitat Management, Honolulu, HI 96850 5 Hawai‘i Cooperative Studies Unit, Pacific Island Ecosystems Research Center, Hawai‘i National Park, HI 96718 6 The Nature Conservancy, Hawai‘i Chapter, Honolulu, HI 96817 7 USDA Natural Resources Conservation Service, Hawaii/Pacific Islands Area State Office, Honolulu, HI 96850 Hawai‘i Cooperative Studies Unit University of Hawai‘i at Hilo 200 W. Kawili St. Hilo, HI 96720 (808) 933-0706 November 2013 This product was prepared under Cooperative Agreement CAG09AC00070 for the Pacific Island Ecosystems Research Center of the U.S. Geological Survey. Technical Report HCSU-044 A LANDSCAPE-BASED ASSESSMENT OF CLIMATE CHANGE VULNERABILITY FOR ALL NATIVE HAWAIIAN PLANTS LUCAS FORTINI1,2, JONATHAN PRICE3, JAMES JACOBI2, ADAM VORSINO4, JEFF BURGETT1,4, KEVIN BRINCK5, FRED AMIDON4, STEVE MILLER4, SAM ʽOHUKANIʽOHIʽA GON III 6, GREGORY KOOB7, AND EBEN PAXTON2 1 Pacific Islands Climate Change Cooperative, Honolulu, HI 96813 2 U.S. Geological Survey, Pacific Island Ecosystems Research Center, Hawaiʽi National Park, HI 96718 3 Department of Geography & Environmental Studies, University of Hawaiʽi at Hilo, Hilo, HI 96720 4 U. -

The Jepson Manual: Vascular Plants of California, Second Edition Supplement II December 2014
The Jepson Manual: Vascular Plants of California, Second Edition Supplement II December 2014 In the pages that follow are treatments that have been revised since the publication of the Jepson eFlora, Revision 1 (July 2013). The information in these revisions is intended to supersede that in the second edition of The Jepson Manual (2012). The revised treatments, as well as errata and other small changes not noted here, are included in the Jepson eFlora (http://ucjeps.berkeley.edu/IJM.html). For a list of errata and small changes in treatments that are not included here, please see: http://ucjeps.berkeley.edu/JM12_errata.html Citation for the entire Jepson eFlora: Jepson Flora Project (eds.) [year] Jepson eFlora, http://ucjeps.berkeley.edu/IJM.html [accessed on month, day, year] Citation for an individual treatment in this supplement: [Author of taxon treatment] 2014. [Taxon name], Revision 2, in Jepson Flora Project (eds.) Jepson eFlora, [URL for treatment]. Accessed on [month, day, year]. Copyright © 2014 Regents of the University of California Supplement II, Page 1 Summary of changes made in Revision 2 of the Jepson eFlora, December 2014 PTERIDACEAE *Pteridaceae key to genera: All of the CA members of Cheilanthes transferred to Myriopteris *Cheilanthes: Cheilanthes clevelandii D. C. Eaton changed to Myriopteris clevelandii (D. C. Eaton) Grusz & Windham, as native Cheilanthes cooperae D. C. Eaton changed to Myriopteris cooperae (D. C. Eaton) Grusz & Windham, as native Cheilanthes covillei Maxon changed to Myriopteris covillei (Maxon) Á. Löve & D. Löve, as native Cheilanthes feei T. Moore changed to Myriopteris gracilis Fée, as native Cheilanthes gracillima D. -
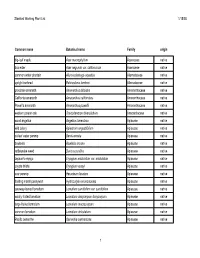
Plant List for Web Page
Stanford Working Plant List 1/15/08 Common name Botanical name Family origin big-leaf maple Acer macrophyllum Aceraceae native box elder Acer negundo var. californicum Aceraceae native common water plantain Alisma plantago-aquatica Alismataceae native upright burhead Echinodorus berteroi Alismataceae native prostrate amaranth Amaranthus blitoides Amaranthaceae native California amaranth Amaranthus californicus Amaranthaceae native Powell's amaranth Amaranthus powellii Amaranthaceae native western poison oak Toxicodendron diversilobum Anacardiaceae native wood angelica Angelica tomentosa Apiaceae native wild celery Apiastrum angustifolium Apiaceae native cutleaf water parsnip Berula erecta Apiaceae native bowlesia Bowlesia incana Apiaceae native rattlesnake weed Daucus pusillus Apiaceae native Jepson's eryngo Eryngium aristulatum var. aristulatum Apiaceae native coyote thistle Eryngium vaseyi Apiaceae native cow parsnip Heracleum lanatum Apiaceae native floating marsh pennywort Hydrocotyle ranunculoides Apiaceae native caraway-leaved lomatium Lomatium caruifolium var. caruifolium Apiaceae native woolly-fruited lomatium Lomatium dasycarpum dasycarpum Apiaceae native large-fruited lomatium Lomatium macrocarpum Apiaceae native common lomatium Lomatium utriculatum Apiaceae native Pacific oenanthe Oenanthe sarmentosa Apiaceae native 1 Stanford Working Plant List 1/15/08 wood sweet cicely Osmorhiza berteroi Apiaceae native mountain sweet cicely Osmorhiza chilensis Apiaceae native Gairdner's yampah (List 4) Perideridia gairdneri gairdneri Apiaceae -

National List of Vascular Plant Species That Occur in Wetlands 1996
National List of Vascular Plant Species that Occur in Wetlands: 1996 National Summary Indicator by Region and Subregion Scientific Name/ North North Central South Inter- National Subregion Northeast Southeast Central Plains Plains Plains Southwest mountain Northwest California Alaska Caribbean Hawaii Indicator Range Abies amabilis (Dougl. ex Loud.) Dougl. ex Forbes FACU FACU UPL UPL,FACU Abies balsamea (L.) P. Mill. FAC FACW FAC,FACW Abies concolor (Gord. & Glend.) Lindl. ex Hildebr. NI NI NI NI NI UPL UPL Abies fraseri (Pursh) Poir. FACU FACU FACU Abies grandis (Dougl. ex D. Don) Lindl. FACU-* NI FACU-* Abies lasiocarpa (Hook.) Nutt. NI NI FACU+ FACU- FACU FAC UPL UPL,FAC Abies magnifica A. Murr. NI UPL NI FACU UPL,FACU Abildgaardia ovata (Burm. f.) Kral FACW+ FAC+ FAC+,FACW+ Abutilon theophrasti Medik. UPL FACU- FACU- UPL UPL UPL UPL UPL NI NI UPL,FACU- Acacia choriophylla Benth. FAC* FAC* Acacia farnesiana (L.) Willd. FACU NI NI* NI NI FACU Acacia greggii Gray UPL UPL FACU FACU UPL,FACU Acacia macracantha Humb. & Bonpl. ex Willd. NI FAC FAC Acacia minuta ssp. minuta (M.E. Jones) Beauchamp FACU FACU Acaena exigua Gray OBL OBL Acalypha bisetosa Bertol. ex Spreng. FACW FACW Acalypha virginica L. FACU- FACU- FAC- FACU- FACU- FACU* FACU-,FAC- Acalypha virginica var. rhomboidea (Raf.) Cooperrider FACU- FAC- FACU FACU- FACU- FACU* FACU-,FAC- Acanthocereus tetragonus (L.) Humm. FAC* NI NI FAC* Acanthomintha ilicifolia (Gray) Gray FAC* FAC* Acanthus ebracteatus Vahl OBL OBL Acer circinatum Pursh FAC- FAC NI FAC-,FAC Acer glabrum Torr. FAC FAC FAC FACU FACU* FAC FACU FACU*,FAC Acer grandidentatum Nutt. -

Chloroplast DNA Evidence for a North American Origin of the Hawaiian
Proc, Natl. Acad. Sci. USA Vol. 88, pp. 1840-1843, March 1991 Evolution Chloroplast DNA evidence for a North American origin of the Hawaiian silversword alliance (Asteraceae) (insular evolution/adaptive radiation/biogeography/long-distance dispersal/phylogenetics) BRUCE G. BALDWIN*t, DONALD W. KYHOS*, JAN DVORAKO, AND GERALD D. CARR§ Departments of *Botany and tAgronomy and Range Science, University of California, Davis, CA 95616; and §Department of Botany, University of Hawaii, Honolulu, HI 96822 Communicated by Peter H. Raven, November 30, 1990 (received for review August 1, 1990) ABSTRACT Chloroplast DNA restriction-site compari- sity, structural (4, 5), biosystematic (6), allozymic (7), and sons were made among 24 species of the Hawaiian silversword chloroplast DNA (cpDNA) (8) data indicate the silversword alliance (Argyroxiphium, Dubautia, and Wilkesia) and 7 species alliance originated from a single colonizing species. of North American perennial tarweeds in Adenothamnus, Ma- Carlquist (1) presented convincing anatomical evidence dia, Raillardella, and Railkrdiopsis (Asteraceae-Madiinae). indicating taxonomic alignment of the Hawaiian silversword These data and results from intergeneric hybridization indi- alliance with the almost exclusively herbaceous American cated surprisingly close genetic affinity of the monophyletic Madiinae or tarweeds. Gray (9) earlier suggested such affinity Hawaiian group to two diploid species of montane perennial for Argyroxiphium, which was disputed by Keck (10) based herbs in California, Madia bolanderi and Raillardiopsis muiri. on presumed morphological dissimilarities and the magnitude Of 117 restriction-site mutations shared among a subset of two of the oceanic barrier to migration. Herein, we compare or more accessions, more than one-fifth (25 mutations) sepa- cpDNA restriction sites between the silversword alliance and rated the silversword alliance, M. -

Wood Anatomy of Duhautia (Asteraceae: Madiinae) in Relation to Adaptive Radiation 1
Pacific Science (1998), vol. 52, no. 4: 356-368 © 1998 by University of Hawai'i Press. All rights reserved Wood Anatomy of Duhautia (Asteraceae: Madiinae) in Relation to Adaptive Radiation 1 SHERWIN CARLQUIST2 ABSTRACT: Qualitative and quantitative features are reported for stem wood of 13 collections of 12 species of the Hawaiian genus Dubautia. Although the species share a basic wood plan, quantitative expressions range widely, espe cially with respect to vessel element dimensions, vessel density, vessel grouping, length of libriform fibers, and dimensions of multiseriate rays. Ecology and habit explain most of the diversity. Variations in the ratio between vessel ele ment length and libriform fiber length are correlated with habit both within Dubautia and when Dubautia is compared with Argyroxiphium and Wilkesia. Other variation in wood is related mostly to ecology. The Dubautia species of wet forest have high mesomorphy ratio values. Low mesomorphy ratio values occur in species of recent or dry lava (e.g., D. scabra) or dry alpine areas (D. menziesii); mesomorphy ratio values in the xeric species are comparable with those in Argyroxiphium. Highly xeromorphic wood in the bog species D. waia lealae may reflect recent immigration from a dry habitat or peculiar features of the bog habitat. The lianoid D. lati/olia has notably xeromorphic wood, which may reflect recent entry into wet forest or else the tendency for lianas in general to have xeromorphic features that confer conductive safety. All species of Dubautia show fiber dimorphism. Dubautia is a superb example of adaptive radiation, in contrast to the Hawaiian Schiedea (Caryophyllaceae), which has shifted into various habitats with little change in wood anatomy, or the Galapagos genus Scalesia, all species of which must survive periods of drought and have xeromorphic wood. -

Herbivorous Insects and the Hawaiian Silversword Alliance: Coevolution Or Cospeciation?L
Pacific Science (1997), vol. 51, no. 4: 440-449 © 1997 by University of Hawai'i Press. All rights reserved Herbivorous Insects and the Hawaiian Silversword Alliance: Coevolution or Cospeciation?l GEORGE K. RODERICK2 ABSTRACT: Numerous groups of herbivorous insects in the Hawaiian archipelago have undergone adaptive radiations. R. C. L. Perkins collected and documented species in nearly all of these groups. In this study I tested whether patterns of host plant use by herbivorous insects can be explained by host plant history. I examined a group ofinsects in the planthopper genus Nesosydne (Hemiptera: Delphacidae) that feed on plants in the Hawaiian silversword alliance, many of which are endangered or threatened. For these Nesosydne species feeding on the silversword alliance, mitochondrial DNA sequence data revealed a statistically significant pattern of cospeciation between these insects and their hosts. These planthoppers are highly host-specific, with each species feeding on only one, or a few closely related, plant species. Patterns ofhost plant use across the plant lineage, as well as within extensive hybrid zones between members of the silversword alliance, suggest that planthopper diversification parallels host plant diversification. Data collected thus far are consis tent with, but do not directly demonstrate, reciprocal adaptation. For other herbivo rous insects associated with members of the Hawaiian silversword alliance, patterns of host plant use and evolutionary history are not yet well understood. However, cospeciation appears not to be universal. For example, endemic flies in the family Tephritidae (Diptera) are less host-specific and demonstrate host-switching. Research is under way to reveal the mechanisms associated with cospeciation and host switching for different insect groups associated with the Hawaiian silversword alliance. -

USDA Forest Service, Pacific Southwest Region Sensitive Plant Species by Forest
USDA Forest Service, Pacific Southwest Region 1 Sensitive Plant Species by Forest 2013 FS R5 RF Plant Species List Klamath NF Mendocino NF Shasta-Trinity NF NF Rivers Six Lassen NF Modoc NF Plumas NF EldoradoNF Inyo NF LTBMU Tahoe NF Sequoia NF Sierra NF Stanislaus NF Angeles NF Cleveland NF Los Padres NF San Bernardino NF Scientific Name (Common Name) Abies bracteata (Santa Lucia fir) X Abronia alpina (alpine sand verbena) X Abronia nana ssp. covillei (Coville's dwarf abronia) X X Abronia villosa var. aurita (chaparral sand verbena) X X Acanthoscyphus parishii var. abramsii (Abrams' flowery puncturebract) X X Acanthoscyphus parishii var. cienegensis (Cienega Seca flowery puncturebract) X Agrostis hooveri (Hoover's bentgrass) X Allium hickmanii (Hickman's onion) X Allium howellii var. clokeyi (Mt. Pinos onion) X Allium jepsonii (Jepson's onion) X X Allium marvinii (Yucaipa onion) X Allium tribracteatum (three-bracted onion) X X Allium yosemitense (Yosemite onion) X X Anisocarpus scabridus (scabrid alpine tarplant) X X X Antennaria marginata (white-margined everlasting) X Antirrhinum subcordatum (dimorphic snapdragon) X Arabis rigidissima var. demota (Carson Range rock cress) X X Arctostaphylos cruzensis (Arroyo de la Cruz manzanita) X Arctostaphylos edmundsii (Little Sur manzanita) X Arctostaphylos glandulosa ssp. gabrielensis (San Gabriel manzanita) X X Arctostaphylos hooveri (Hoover's manzanita) X Arctostaphylos luciana (Santa Lucia manzanita) X Arctostaphylos nissenana (Nissenan manzanita) X X Arctostaphylos obispoensis (Bishop manzanita) X Arctostphylos parryana subsp. tumescens (interior manzanita) X X Arctostaphylos pilosula (Santa Margarita manzanita) X Arctostaphylos rainbowensis (rainbow manzanita) X Arctostaphylos refugioensis (Refugio manzanita) X Arenaria lanuginosa ssp. saxosa (rock sandwort) X Astragalus anxius (Ash Valley milk-vetch) X Astragalus bernardinus (San Bernardino milk-vetch) X Astragalus bicristatus (crested milk-vetch) X X Pacific Southwest Region, Regional Forester's Sensitive Species List. -

THE STATUS of the MAUNA KEA SILVERSWORD Gerald D. Carr And
34 THE STATUS OF THE MAUNA KEA SILVERSWORD Gerald D. Carr and Alain Meyrat Department of Botany University of Hawaii 3190 Maile Way Honolulu, Hawaii 96822 The Mauna Kea silversword was first brought to the attention of the scientific community through the efforts of Scottish botanist James M~crae. Macrae ascended Mauna Kea from Laupahoehoe in 1825 and at a point near the summit, after walking three miles over sandy pulverized lava, noted, "The last mile was destitute of vegetation except one plant of the Syginesia tribe, in growth much like a Yucca, with sharp pointed silver cou1oured leaves and green upright spike of three or four feet producing pendulous branches with br9wn flowers, truly superb, and almost worth the journey of coming here to s~e it on purpose" (Wilson 1922, p. 54). Specimens of t;hese 'truly superb' plants reached De Candolle and in 1836 were christened Argyroxiphium sandwicense DC. Early in 1834, David Douglas ascended Mauna Kea and observed the same species, specimens of which reached W. J. Hooker and were initially given the name Argyrophyton douglasii Hook. (1837a.), but Hooker very soon a.ccepted De Candolle's earlier name for the taxon (Hooker 1837b). ,The species was again collected on Mauna Kea by Charles Pickering in 1841 as part of the U. S. South Pacific Exploring Expedition (cf. Pickering 1876, Keck 1936). Pickering also collected material he presumed to belong to the Same taxon on Haleakala, Maui, but this was considered distinct by Asa Gray and was named Argyroxiphium macrocephalum (cf. Gray 1852, Pickering 1876). Both Pickering and Douglas observed Argyroxiphium on Mauna Loa and Mauna Kea, Hawai'i and considered them to be the same taxon (Pickering 1876, Wilson 1922).