Supplementary Figures
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Supplementary Table 1: Adhesion Genes Data Set
Supplementary Table 1: Adhesion genes data set PROBE Entrez Gene ID Celera Gene ID Gene_Symbol Gene_Name 160832 1 hCG201364.3 A1BG alpha-1-B glycoprotein 223658 1 hCG201364.3 A1BG alpha-1-B glycoprotein 212988 102 hCG40040.3 ADAM10 ADAM metallopeptidase domain 10 133411 4185 hCG28232.2 ADAM11 ADAM metallopeptidase domain 11 110695 8038 hCG40937.4 ADAM12 ADAM metallopeptidase domain 12 (meltrin alpha) 195222 8038 hCG40937.4 ADAM12 ADAM metallopeptidase domain 12 (meltrin alpha) 165344 8751 hCG20021.3 ADAM15 ADAM metallopeptidase domain 15 (metargidin) 189065 6868 null ADAM17 ADAM metallopeptidase domain 17 (tumor necrosis factor, alpha, converting enzyme) 108119 8728 hCG15398.4 ADAM19 ADAM metallopeptidase domain 19 (meltrin beta) 117763 8748 hCG20675.3 ADAM20 ADAM metallopeptidase domain 20 126448 8747 hCG1785634.2 ADAM21 ADAM metallopeptidase domain 21 208981 8747 hCG1785634.2|hCG2042897 ADAM21 ADAM metallopeptidase domain 21 180903 53616 hCG17212.4 ADAM22 ADAM metallopeptidase domain 22 177272 8745 hCG1811623.1 ADAM23 ADAM metallopeptidase domain 23 102384 10863 hCG1818505.1 ADAM28 ADAM metallopeptidase domain 28 119968 11086 hCG1786734.2 ADAM29 ADAM metallopeptidase domain 29 205542 11085 hCG1997196.1 ADAM30 ADAM metallopeptidase domain 30 148417 80332 hCG39255.4 ADAM33 ADAM metallopeptidase domain 33 140492 8756 hCG1789002.2 ADAM7 ADAM metallopeptidase domain 7 122603 101 hCG1816947.1 ADAM8 ADAM metallopeptidase domain 8 183965 8754 hCG1996391 ADAM9 ADAM metallopeptidase domain 9 (meltrin gamma) 129974 27299 hCG15447.3 ADAMDEC1 ADAM-like, -

Journal of Pediatric Gastroenterology and Nutrition, Publish Ahead of Print
Journal of Pediatric Gastroenterology and Nutrition, Publish Ahead of Print DOI : 10.1097/MPG.0000000000002462 Serologic, but not genetic, markers are associated with impaired anthropometrics at diagnosis of pediatric Crohn’s disease Authors: Sara K. Naramore, MD1, William E. Bennett, Jr., MD, MS1,2, Guanglong Jiang, MS3,4, Subra Kugathasan, MD5, Lee A. Denson, MD6, Jeffrey S. Hyams, MD7, Steven J. Steiner, MD1, and PRO-KIIDS Research Group8† 1Department of Pediatrics, Division of Pediatric Gastroenterology, Hepatology, and Nutrition, Indiana University School of Medicine, Indianapolis, IN 2Department of Pediatrics, Division of Pediatric and Adolescent Comparative Effectiveness Research, Indiana University School of Medicine, Indianapolis, IN 3Department of Medical & Molecular Genetics, Indiana University School of Medicine, Indianapolis, IN 4Department of BioHealth Informatics, Indiana University-Purdue University−Indianapolis, Indianapolis, IN 5Department of Pediatrics, Emory University School of Medicine, Atlanta, GA 6Department of Pediatrics, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH 7Department of Pediatrics, Connecticut Children’s Medical Center, Hartford, CT 8PRO-KIIDS Research Group, New York, NY †Membership of the PRO-KIIDS Research Group is listed in the Acknowledgements. Principal Investigator and Corresponding Author: Sara Naramore, MD Department of Pediatrics Indiana University School of Medicine Riley Hospital for Children ____________________________________________________ This is the author's manuscript of the article published in final edited form as: Naramore, S. K., Bennett, W. E. J., Jiang, G., Kugathasan, S., Denson, L. A., Hyams, J. S., … Group, and P.-K. R. (2019). Serologic, but not Genetic, Markers are Associated with Impaired Anthropometrics at Diagnosis of Pediatric Crohn’s Disease. Journal of Pediatric Gastroenterology and Nutrition, Publish Ahead of Print. -

NICU Gene List Generator.Xlsx
Neonatal Crisis Sequencing Panel Gene List Genes: A2ML1 - B3GLCT A2ML1 ADAMTS9 ALG1 ARHGEF15 AAAS ADAMTSL2 ALG11 ARHGEF9 AARS1 ADAR ALG12 ARID1A AARS2 ADARB1 ALG13 ARID1B ABAT ADCY6 ALG14 ARID2 ABCA12 ADD3 ALG2 ARL13B ABCA3 ADGRG1 ALG3 ARL6 ABCA4 ADGRV1 ALG6 ARMC9 ABCB11 ADK ALG8 ARPC1B ABCB4 ADNP ALG9 ARSA ABCC6 ADPRS ALK ARSL ABCC8 ADSL ALMS1 ARX ABCC9 AEBP1 ALOX12B ASAH1 ABCD1 AFF3 ALOXE3 ASCC1 ABCD3 AFF4 ALPK3 ASH1L ABCD4 AFG3L2 ALPL ASL ABHD5 AGA ALS2 ASNS ACAD8 AGK ALX3 ASPA ACAD9 AGL ALX4 ASPM ACADM AGPS AMELX ASS1 ACADS AGRN AMER1 ASXL1 ACADSB AGT AMH ASXL3 ACADVL AGTPBP1 AMHR2 ATAD1 ACAN AGTR1 AMN ATL1 ACAT1 AGXT AMPD2 ATM ACE AHCY AMT ATP1A1 ACO2 AHDC1 ANK1 ATP1A2 ACOX1 AHI1 ANK2 ATP1A3 ACP5 AIFM1 ANKH ATP2A1 ACSF3 AIMP1 ANKLE2 ATP5F1A ACTA1 AIMP2 ANKRD11 ATP5F1D ACTA2 AIRE ANKRD26 ATP5F1E ACTB AKAP9 ANTXR2 ATP6V0A2 ACTC1 AKR1D1 AP1S2 ATP6V1B1 ACTG1 AKT2 AP2S1 ATP7A ACTG2 AKT3 AP3B1 ATP8A2 ACTL6B ALAS2 AP3B2 ATP8B1 ACTN1 ALB AP4B1 ATPAF2 ACTN2 ALDH18A1 AP4M1 ATR ACTN4 ALDH1A3 AP4S1 ATRX ACVR1 ALDH3A2 APC AUH ACVRL1 ALDH4A1 APTX AVPR2 ACY1 ALDH5A1 AR B3GALNT2 ADA ALDH6A1 ARFGEF2 B3GALT6 ADAMTS13 ALDH7A1 ARG1 B3GAT3 ADAMTS2 ALDOB ARHGAP31 B3GLCT Updated: 03/15/2021; v.3.6 1 Neonatal Crisis Sequencing Panel Gene List Genes: B4GALT1 - COL11A2 B4GALT1 C1QBP CD3G CHKB B4GALT7 C3 CD40LG CHMP1A B4GAT1 CA2 CD59 CHRNA1 B9D1 CA5A CD70 CHRNB1 B9D2 CACNA1A CD96 CHRND BAAT CACNA1C CDAN1 CHRNE BBIP1 CACNA1D CDC42 CHRNG BBS1 CACNA1E CDH1 CHST14 BBS10 CACNA1F CDH2 CHST3 BBS12 CACNA1G CDK10 CHUK BBS2 CACNA2D2 CDK13 CILK1 BBS4 CACNB2 CDK5RAP2 -

Gene Symbol Category ACAN ECM ADAM10 ECM Remodeling-Related ADAM11 ECM Remodeling-Related ADAM12 ECM Remodeling-Related ADAM15 E
Supplementary Material (ESI) for Integrative Biology This journal is (c) The Royal Society of Chemistry 2010 Gene symbol Category ACAN ECM ADAM10 ECM remodeling-related ADAM11 ECM remodeling-related ADAM12 ECM remodeling-related ADAM15 ECM remodeling-related ADAM17 ECM remodeling-related ADAM18 ECM remodeling-related ADAM19 ECM remodeling-related ADAM2 ECM remodeling-related ADAM20 ECM remodeling-related ADAM21 ECM remodeling-related ADAM22 ECM remodeling-related ADAM23 ECM remodeling-related ADAM28 ECM remodeling-related ADAM29 ECM remodeling-related ADAM3 ECM remodeling-related ADAM30 ECM remodeling-related ADAM5 ECM remodeling-related ADAM7 ECM remodeling-related ADAM8 ECM remodeling-related ADAM9 ECM remodeling-related ADAMTS1 ECM remodeling-related ADAMTS10 ECM remodeling-related ADAMTS12 ECM remodeling-related ADAMTS13 ECM remodeling-related ADAMTS14 ECM remodeling-related ADAMTS15 ECM remodeling-related ADAMTS16 ECM remodeling-related ADAMTS17 ECM remodeling-related ADAMTS18 ECM remodeling-related ADAMTS19 ECM remodeling-related ADAMTS2 ECM remodeling-related ADAMTS20 ECM remodeling-related ADAMTS3 ECM remodeling-related ADAMTS4 ECM remodeling-related ADAMTS5 ECM remodeling-related ADAMTS6 ECM remodeling-related ADAMTS7 ECM remodeling-related ADAMTS8 ECM remodeling-related ADAMTS9 ECM remodeling-related ADAMTSL1 ECM remodeling-related ADAMTSL2 ECM remodeling-related ADAMTSL3 ECM remodeling-related ADAMTSL4 ECM remodeling-related ADAMTSL5 ECM remodeling-related AGRIN ECM ALCAM Cell-cell adhesion ANGPT1 Soluble factors and receptors -

Dock3 Stimulates Axonal Outgrowth Via GSK-3ß-Mediated Microtubule
264 • The Journal of Neuroscience, January 4, 2012 • 32(1):264–274 Development/Plasticity/Repair Dock3 Stimulates Axonal Outgrowth via GSK-3-Mediated Microtubule Assembly Kazuhiko Namekata, Chikako Harada, Xiaoli Guo, Atsuko Kimura, Daiji Kittaka, Hayaki Watanabe, and Takayuki Harada Visual Research Project, Tokyo Metropolitan Institute of Medical Science, Tokyo 156-8506, Japan Dock3, a new member of the guanine nucleotide exchange factors, causes cellular morphological changes by activating the small GTPase Rac1. Overexpression of Dock3 in neural cells promotes axonal outgrowth downstream of brain-derived neurotrophic factor (BDNF) signaling. We previously showed that Dock3 forms a complex with Fyn and WASP (Wiskott–Aldrich syndrome protein) family verprolin- homologous (WAVE) proteins at the plasma membrane, and subsequent Rac1 activation promotes actin polymerization. Here we show that Dock3 binds to and inactivates glycogen synthase kinase-3 (GSK-3) at the plasma membrane, thereby increasing the nonphos- phorylated active form of collapsin response mediator protein-2 (CRMP-2), which promotes axon branching and microtubule assembly. Exogenously applied BDNF induced the phosphorylation of GSK-3 and dephosphorylation of CRMP-2 in hippocampal neurons. More- over, increased phosphorylation of GSK-3 was detected in the regenerating axons of transgenic mice overexpressing Dock3 after optic nerve injury. These results suggest that Dock3 plays important roles downstream of BDNF signaling in the CNS, where it regulates cell polarity and promotes axonal outgrowth by stimulating dual pathways: actin polymerization and microtubule assembly. Introduction We recently detected a common active center of Dock1ϳ4 within The Rho-GTPases, including Rac1, Cdc42, and RhoA, are best the DHR-2 domain and reported that the DHR-1 domain is nec- known for their roles in regulating the actin cytoskeleton and are essary for the direct binding between Dock1ϳ4 and WAVE1ϳ3 implicated in a broad spectrum of biological functions, such as (Namekata et al., 2010). -
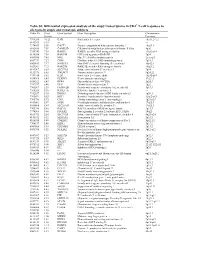
Table S1| Differential Expression Analysis of the Atopy Transcriptome
Table S1| Differential expression analysis of the atopy transcriptome in CD4+ T-cell responses to allergens in atopic and nonatopic subjects Probe ID S.test Gene Symbol Gene Description Chromosome Statistic Location 7994280 10.32 IL4R Interleukin 4 receptor 16p11.2-12.1 8143383 8.95 --- --- --- 7974689 8.50 DACT1 Dapper, antagonist of beta-catenin, homolog 1 14q23.1 8102415 7.59 CAMK2D Calcium/calmodulin-dependent protein kinase II delta 4q26 7950743 7.58 RAB30 RAB30, member RAS oncogene family 11q12-q14 8136580 7.54 RAB19B GTP-binding protein RAB19B 7q34 8043504 7.45 MAL Mal, T-cell differentiation protein 2cen-q13 8087739 7.27 CISH Cytokine inducible SH2-containing protein 3p21.3 8000413 7.17 NSMCE1 Non-SMC element 1 homolog (S. cerevisiae) 16p12.1 8021301 7.15 RAB27B RAB27B, member RAS oncogene family 18q21.2 8143367 6.83 SLC37A3 Solute carrier family 37 member 3 7q34 8152976 6.65 TMEM71 Transmembrane protein 71 8q24.22 7931914 6.56 IL2R Interleukin 2 receptor, alpha 10p15-p14 8014768 6.43 PLXDC1 Plexin domain containing 1 17q21.1 8056222 6.43 DPP4 Dipeptidyl-peptidase 4 (CD26) 2q24.3 7917697 6.40 GFI1 Growth factor independent 1 1p22 7903507 6.39 FAM102B Family with sequence similarity 102, member B 1p13.3 7968236 5.96 RASL11A RAS-like, family 11, member A --- 7912537 5.95 DHRS3 Dehydrogenase/reductase (SDR family) member 3 1p36.1 7963491 5.83 KRT1 Keratin 1 (epidermolytic hyperkeratosis) 12q12-q13 7903786 5.72 CSF1 Colony stimulating factor 1 (macrophage) 1p21-p13 8019061 5.67 SGSH N-sulfoglucosamine sulfohydrolase (sulfamidase) 17q25.3 -

Identification of Potential Key Genes and Pathway Linked with Sporadic Creutzfeldt-Jakob Disease Based on Integrated Bioinformatics Analyses
medRxiv preprint doi: https://doi.org/10.1101/2020.12.21.20248688; this version posted December 24, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted medRxiv a license to display the preprint in perpetuity. All rights reserved. No reuse allowed without permission. Identification of potential key genes and pathway linked with sporadic Creutzfeldt-Jakob disease based on integrated bioinformatics analyses Basavaraj Vastrad1, Chanabasayya Vastrad*2 , Iranna Kotturshetti 1. Department of Biochemistry, Basaveshwar College of Pharmacy, Gadag, Karnataka 582103, India. 2. Biostatistics and Bioinformatics, Chanabasava Nilaya, Bharthinagar, Dharwad 580001, Karanataka, India. 3. Department of Ayurveda, Rajiv Gandhi Education Society`s Ayurvedic Medical College, Ron, Karnataka 562209, India. * Chanabasayya Vastrad [email protected] Ph: +919480073398 Chanabasava Nilaya, Bharthinagar, Dharwad 580001 , Karanataka, India NOTE: This preprint reports new research that has not been certified by peer review and should not be used to guide clinical practice. medRxiv preprint doi: https://doi.org/10.1101/2020.12.21.20248688; this version posted December 24, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted medRxiv a license to display the preprint in perpetuity. All rights reserved. No reuse allowed without permission. Abstract Sporadic Creutzfeldt-Jakob disease (sCJD) is neurodegenerative disease also called prion disease linked with poor prognosis. The aim of the current study was to illuminate the underlying molecular mechanisms of sCJD. The mRNA microarray dataset GSE124571 was downloaded from the Gene Expression Omnibus database. Differentially expressed genes (DEGs) were screened. -

Lfp Cv May 2021 1
Curriculum Vitae Luis F. Parada, Ph.D. [email protected] 1275 York Avenue, Box 558 New York, NY 10065 T 646-888-3781 www.mskcc.org Education & positions held 1979 B.S., Molecular Biology (with Honors), University of Wisconsin-Madison, Wisconsin 1985 Ph.D., Biology, Massachusetts Institute of Technology, Cambridge, Massachusetts 1985 - 1987 Postdoctoral Fellow, Unité de Génetique Cellulaire, Pasteur Institute, Paris, France 1988 - 1994 Head, Molecular Embryology Group & Molecular Embryology Section (with tenure), Mammalian Genetics Laboratory, ABL-Basic Research Program, NCI-Frederick Cancer Research and Development Center, Frederick, Maryland 1994 - 2006 Director, Center for Developmental Biology and Professor of Cell Biology University of Texas Southwestern Medical Center, Dallas, TX 1995 - 2015 Diana and Richard C. Strauss Distinguished Chair in Developmental Biology 1997 - 2015 Director, Kent Waldrep Center for Basic Research on Nerve Growth and Regeneration 1998 - 2015 Southwestern Ball Distinguished Chair in Basic Neuroscience Research 2003 - American Cancer Society Research Professor 2006 - 2015 Chairman, Department of Developmental Biology, University of Texas Southwestern Medical School 2015- Director, Brain Tumor Center & Member Cancer Biology and Genetics Program, SKI & MSKCC 2015- Albert C. Foster Chair, SKI & MSKCC 2015- Attending Neuroscientist, Departments of Neurology and Neurosurgery, MSKCC Luis F. Parada Page 2 of 28 Honors 2018 EACR Keynote Lecture. The 4th Brain Tumours 2018: From Biology to Therapy Conference. Warsaw, Poland. 2018 Keynote Lecture CRUK Brain Tumour Conference 2018. London 2016 - 2023 NCI Outstanding Investigator Award (R35) 2015 Distinguished Lectureship in Cancer Biology, MD Anderson 2014 The Maestro Award – Dallas, TX 2014 The Herman Vanden Berghe Lectures – University of Leuven, Belgium 2013 Blaffer Lecture – M.D. -

Whole Exome Sequencing in Families at High Risk for Hodgkin Lymphoma: Identification of a Predisposing Mutation in the KDR Gene
Hodgkin Lymphoma SUPPLEMENTARY APPENDIX Whole exome sequencing in families at high risk for Hodgkin lymphoma: identification of a predisposing mutation in the KDR gene Melissa Rotunno, 1 Mary L. McMaster, 1 Joseph Boland, 2 Sara Bass, 2 Xijun Zhang, 2 Laurie Burdett, 2 Belynda Hicks, 2 Sarangan Ravichandran, 3 Brian T. Luke, 3 Meredith Yeager, 2 Laura Fontaine, 4 Paula L. Hyland, 1 Alisa M. Goldstein, 1 NCI DCEG Cancer Sequencing Working Group, NCI DCEG Cancer Genomics Research Laboratory, Stephen J. Chanock, 5 Neil E. Caporaso, 1 Margaret A. Tucker, 6 and Lynn R. Goldin 1 1Genetic Epidemiology Branch, Division of Cancer Epidemiology and Genetics, National Cancer Institute, NIH, Bethesda, MD; 2Cancer Genomics Research Laboratory, Division of Cancer Epidemiology and Genetics, National Cancer Institute, NIH, Bethesda, MD; 3Ad - vanced Biomedical Computing Center, Leidos Biomedical Research Inc.; Frederick National Laboratory for Cancer Research, Frederick, MD; 4Westat, Inc., Rockville MD; 5Division of Cancer Epidemiology and Genetics, National Cancer Institute, NIH, Bethesda, MD; and 6Human Genetics Program, Division of Cancer Epidemiology and Genetics, National Cancer Institute, NIH, Bethesda, MD, USA ©2016 Ferrata Storti Foundation. This is an open-access paper. doi:10.3324/haematol.2015.135475 Received: August 19, 2015. Accepted: January 7, 2016. Pre-published: June 13, 2016. Correspondence: [email protected] Supplemental Author Information: NCI DCEG Cancer Sequencing Working Group: Mark H. Greene, Allan Hildesheim, Nan Hu, Maria Theresa Landi, Jennifer Loud, Phuong Mai, Lisa Mirabello, Lindsay Morton, Dilys Parry, Anand Pathak, Douglas R. Stewart, Philip R. Taylor, Geoffrey S. Tobias, Xiaohong R. Yang, Guoqin Yu NCI DCEG Cancer Genomics Research Laboratory: Salma Chowdhury, Michael Cullen, Casey Dagnall, Herbert Higson, Amy A. -

(A) TCGA-LUAD, (B) TCGA-LUSC, (C) Database from Rizvi H, Et Al
Figure S1. The correlations of TMB value between panel sequencing and WES in published databases. (A) TCGA-LUAD, (B) TCGA-LUSC, (C) Database from Rizvi H, et al. J Clin Oncol, 2018;36. Abbreviations: TMB, tumor mutational burden; TCGA, The Cancer Genome Atlas; LUAD, lung adenocarcinoma; LUSC, lung squamous cell carcinoma; WES, wholeexome sequencing A B C 1 / 3 Figure S2. The comparison of multi-region tTMB among different NSCLC subtypes. Abbreviations: TMB, tumor mutational burden; tTMB, tissue TMB 2 / 3 Figure S3. The comparisons and overlaps of tumor-derived mutational profiles among tumor tissues in each region and the corresponding ctDNA. The P0XX was the patient No. shown at the top. Each tumor region (T1, T2, T3…) with plasma (P) were arranged in the x axis. ctDNA was isolated from plasma (more details in Supplementary Methods). Right y axis displayed tumor-derived mutational profiles in detail. The detected mutations were shown in red, while undetected cases were shown in gray. 3 / 3 P001 P004 P005 P006 P007 P009 P015 P018 KRAS.p.G12C ATG9B.p.R298C MORC1.p.G97V MLH1.p.L658V EGFR.p.L747_E749del SPTA1.p.S444C VAV1.p.R548W TP53.p.Y163C BAX.p.E41G CSMD3.p.H1455Y FAT1.p.R4481L CHI3L1.p.G37S DDR2.p.R478C APC.p.E2184K EPOR.p.R45P PTCH2.p.E854K TENM3.p.S44I EGFR.p.L858R U2AF1.p.S34F STK11.p.I238F MAEL.c.703.1G.C PIK3CA.p.R108H MSH6.p.S1340C EGFR.p.E746_A750del TSC1.p.R779. FLT1.p.K37E XRCC1.p.E455Q TP53.c.443.2A.C EGFR.p.A750P PPEF1.p.R348Q NTRK3.p.D208E TP53.p.Q105_S110delinsP DNMT3A.p.F563I NF1.p.A1228T CDK13.p.K852Nfs.23 POLR3B.c.1102.3_1102.2delTA COL6A6.p.P1542S MYD88.p.L265P FLT4.p.A601V TERT.c..58.u3403C.A ACIN1.p.K1047N ERBB2.p.G746delinsVC PRKCD.p.G210V EPHA3.p.T519M NOTCH1.p.Q2361. -

X-Linked Diseases: Susceptible Females
REVIEW ARTICLE X-linked diseases: susceptible females Barbara R. Migeon, MD 1 The role of X-inactivation is often ignored as a prime cause of sex data include reasons why women are often protected from the differences in disease. Yet, the way males and females express their deleterious variants carried on their X chromosome, and the factors X-linked genes has a major role in the dissimilar phenotypes that that render women susceptible in some instances. underlie many rare and common disorders, such as intellectual deficiency, epilepsy, congenital abnormalities, and diseases of the Genetics in Medicine (2020) 22:1156–1174; https://doi.org/10.1038/s41436- heart, blood, skin, muscle, and bones. Summarized here are many 020-0779-4 examples of the different presentations in males and females. Other INTRODUCTION SEX DIFFERENCES ARE DUE TO X-INACTIVATION Sex differences in human disease are usually attributed to The sex differences in the effect of X-linked pathologic variants sex specific life experiences, and sex hormones that is due to our method of X chromosome dosage compensation, influence the function of susceptible genes throughout the called X-inactivation;9 humans and most placental mammals – genome.1 5 Such factors do account for some dissimilarities. compensate for the sex difference in number of X chromosomes However, a major cause of sex-determined expression of (that is, XX females versus XY males) by transcribing only one disease has to do with differences in how males and females of the two female X chromosomes. X-inactivation silences all X transcribe their gene-rich human X chromosomes, which is chromosomes but one; therefore, both males and females have a often underappreciated as a cause of sex differences in single active X.10,11 disease.6 Males are the usual ones affected by X-linked For 46 XY males, that X is the only one they have; it always pathogenic variants.6 Females are biologically superior; a comes from their mother, as fathers contribute their Y female usually has no disease, or much less severe disease chromosome. -

Supplementary Table 2
Supplementary Table 2. Differentially Expressed Genes following Sham treatment relative to Untreated Controls Fold Change Accession Name Symbol 3 h 12 h NM_013121 CD28 antigen Cd28 12.82 BG665360 FMS-like tyrosine kinase 1 Flt1 9.63 NM_012701 Adrenergic receptor, beta 1 Adrb1 8.24 0.46 U20796 Nuclear receptor subfamily 1, group D, member 2 Nr1d2 7.22 NM_017116 Calpain 2 Capn2 6.41 BE097282 Guanine nucleotide binding protein, alpha 12 Gna12 6.21 NM_053328 Basic helix-loop-helix domain containing, class B2 Bhlhb2 5.79 NM_053831 Guanylate cyclase 2f Gucy2f 5.71 AW251703 Tumor necrosis factor receptor superfamily, member 12a Tnfrsf12a 5.57 NM_021691 Twist homolog 2 (Drosophila) Twist2 5.42 NM_133550 Fc receptor, IgE, low affinity II, alpha polypeptide Fcer2a 4.93 NM_031120 Signal sequence receptor, gamma Ssr3 4.84 NM_053544 Secreted frizzled-related protein 4 Sfrp4 4.73 NM_053910 Pleckstrin homology, Sec7 and coiled/coil domains 1 Pscd1 4.69 BE113233 Suppressor of cytokine signaling 2 Socs2 4.68 NM_053949 Potassium voltage-gated channel, subfamily H (eag- Kcnh2 4.60 related), member 2 NM_017305 Glutamate cysteine ligase, modifier subunit Gclm 4.59 NM_017309 Protein phospatase 3, regulatory subunit B, alpha Ppp3r1 4.54 isoform,type 1 NM_012765 5-hydroxytryptamine (serotonin) receptor 2C Htr2c 4.46 NM_017218 V-erb-b2 erythroblastic leukemia viral oncogene homolog Erbb3 4.42 3 (avian) AW918369 Zinc finger protein 191 Zfp191 4.38 NM_031034 Guanine nucleotide binding protein, alpha 12 Gna12 4.38 NM_017020 Interleukin 6 receptor Il6r 4.37 AJ002942