61/2003, Uredbeni
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Pp375-430-Annex 1.Qxd
ANNEX 1 CHEMICAL AND PHYSICAL DATA ON COMPOUNDS USED IN COMBINED ESTROGEN–PROGESTOGEN CONTRACEPTIVES AND HORMONAL MENOPAUSAL THERAPY Annex 1 describes the chemical and physical data, technical products, trends in produc- tion by region and uses of estrogens and progestogens in combined estrogen–progestogen contraceptives and hormonal menopausal therapy. Estrogens and progestogens are listed separately in alphabetical order. Trade names for these compounds alone and in combination are given in Annexes 2–4. Sales are listed according to the regions designated by WHO. These are: Africa: Algeria, Angola, Benin, Botswana, Burkina Faso, Burundi, Cameroon, Cape Verde, Central African Republic, Chad, Comoros, Congo, Côte d'Ivoire, Democratic Republic of the Congo, Equatorial Guinea, Eritrea, Ethiopia, Gabon, Gambia, Ghana, Guinea, Guinea-Bissau, Kenya, Lesotho, Liberia, Madagascar, Malawi, Mali, Mauritania, Mauritius, Mozambique, Namibia, Niger, Nigeria, Rwanda, Sao Tome and Principe, Senegal, Seychelles, Sierra Leone, South Africa, Swaziland, Togo, Uganda, United Republic of Tanzania, Zambia and Zimbabwe America (North): Canada, Central America (Antigua and Barbuda, Bahamas, Barbados, Belize, Costa Rica, Cuba, Dominica, El Salvador, Grenada, Guatemala, Haiti, Honduras, Jamaica, Mexico, Nicaragua, Panama, Puerto Rico, Saint Kitts and Nevis, Saint Lucia, Saint Vincent and the Grenadines, Suriname, Trinidad and Tobago), United States of America America (South): Argentina, Bolivia, Brazil, Chile, Colombia, Dominican Republic, Ecuador, Guyana, Paraguay, -

MMC International BV
M.M.C. International Steroid Substances Steroid Test A Colour Steroid Test B Colour Steroid Test B Colour with UV Light Stanozolol/ Oxandrolone Test Clenbuterol/ Oxymetholone Test Ephedrine Test Alfadolone Orange Yellow Nil - - - Androsterone Orange Yellow White - - - Beclometasone Brown–yellow Orange Nil - - - Betamethasone Orange–brown Pink–Orange Nil - - - Boldenone Base (Equipoise, Ganabol) (pure powder) Warm red after 2 min. Dark Orange after 2 min. Bright Light Orange - - - Boldenone Undecanoate (oil) Dark brownish-red Dark Red Bright Light Orange - - - Boldenone Undecylenate (oil) Orange - Light Brown Dark Orange → Brown Bright Light Orange-Yellow - - - Carbenoxolone (CBX) Orange Yellow Yellow - - - Cholesterol Violet Orange White - - - Clenbuterol (Spiropent, Ventipulmin) - - - - Purple - Dark brown with yellow-green on the Dark brown with yellow-green on the Clomiphene (Androxal, Clomid, Omifin) Nil Dark brown to black No reaction Dark brown to black sides of the ampoule sides of the ampoule Cortisone Orange Yellow Green - - - Desoxycortone Blue–black Yellow Yellow - - - Dexamethasone Yellow Orange–pink Nil - - - Dienestrol Yellow Orange–red Nil - - - Diethylstilbestrol (DES) Orange (→yellow–green) Nil - - - Dimethisterone Brown–green Orange–red Yellow - - - Drostanolone Propionate (Masteron) (oil) Bright green Yellow-Orange Orange - - - Dydrogesterone (Duphaston) - Orange Green-Yellow - - - Enoxolone Orange Yellow Green-Yellow - - - Ephedrine (also for Pseudo- and Nor-Ephedrine) - - - - - Orange Estradiol (Oestradiol) Orange -

Combined Estrogen–Progestogen Menopausal Therapy
COMBINED ESTROGEN–PROGESTOGEN MENOPAUSAL THERAPY Combined estrogen–progestogen menopausal therapy was considered by previous IARC Working Groups in 1998 and 2005 (IARC, 1999, 2007). Since that time, new data have become available, these have been incorporated into the Monograph, and taken into consideration in the present evaluation. 1. Exposure Data 1.1.2 Progestogens (a) Chlormadinone acetate Combined estrogen–progestogen meno- Chem. Abstr. Serv. Reg. No.: 302-22-7 pausal therapy involves the co-administration Chem. Abstr. Name: 17-(Acetyloxy)-6-chlo- of an estrogen and a progestogen to peri- or ropregna-4,6-diene-3,20-dione menopausal women. The use of estrogens with IUPAC Systematic Name: 6-Chloro-17-hy- progestogens has been recommended to prevent droxypregna-4,6-diene-3,20-dione, acetate the estrogen-associated risk of endometrial Synonyms: 17α-Acetoxy-6-chloro-4,6- cancer. Evidence from the Women’s Health pregnadiene-3,20-dione; 6-chloro-Δ6-17- Initiative (WHI) of adverse effects from the use acetoxyprogesterone; 6-chloro-Δ6-[17α] of a continuous combined estrogen–progestogen acetoxyprogesterone has affected prescribing. Patterns of exposure Structural and molecular formulae, and relative are also changing rapidly as the use of hormonal molecular mass therapy declines, the indications are restricted, O CH and the duration of the therapy is reduced (IARC, 3 C 2007). CH3 CH3 O C 1.1 Identification of the agents CH3 H O 1.1.1 Estrogens HH For Estrogens, see the Monograph on O Estrogen-only Menopausal Therapy in this Cl volume. C23H29ClO4 Relative molecular mass: 404.9 249 IARC MONOGRAPHS – 100A (b) Cyproterone acetate Structural and molecular formulae, and relative Chem. -

(ESI) for Integrative Biology. This Journal Is © the Royal Society of Chemistry 2017
Electronic Supplementary Material (ESI) for Integrative Biology. This journal is © The Royal Society of Chemistry 2017 Table 1 Enriched GO terms with p-value ≤ 0.05 corresponding to the over-expressed genes upon perturbation with the lung-toxic compounds. Terms with corrected p-value less than 0.001 are shown in bold. GO:0043067 regulation of programmed GO:0010941 regulation of cell death cell death GO:0042981 regulation of apoptosis GO:0010033 response to organic sub- stance GO:0043068 positive regulation of pro- GO:0010942 positive regulation of cell grammed cell death death GO:0006357 regulation of transcription GO:0043065 positive regulation of apop- from RNA polymerase II promoter tosis GO:0010035 response to inorganic sub- GO:0043066 negative regulation of stance apoptosis GO:0043069 negative regulation of pro- GO:0060548 negative regulation of cell death grammed cell death GO:0016044 membrane organization GO:0042592 homeostatic process GO:0010629 negative regulation of gene ex- GO:0001568 blood vessel development pression GO:0051172 negative regulation of nitrogen GO:0006468 protein amino acid phosphoryla- compound metabolic process tion GO:0070482 response to oxygen levels GO:0045892 negative regulation of transcrip- tion, DNA-dependent GO:0001944 vasculature development GO:0046907 intracellular transport GO:0008202 steroid metabolic process GO:0045934 negative regulation of nucle- obase, nucleoside, nucleotide and nucleic acid metabolic process GO:0006917 induction of apoptosis GO:0016481 negative regulation of transcrip- tion GO:0016125 sterol metabolic process GO:0012502 induction of programmed cell death GO:0001666 response to hypoxia GO:0051253 negative regulation of RNA metabolic process GO:0008203 cholesterol metabolic process GO:0010551 regulation of specific transcrip- tion from RNA polymerase II promoter 1 Table 2 Enriched GO terms with p-value ≤ 0.05 corresponding to the under-expressed genes upon perturbation with the lung-toxic compounds. -
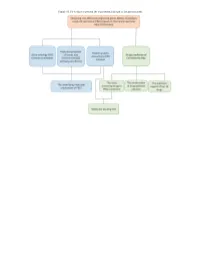
Figure S1. Flow Chart to Present the Experimental Design of the Present Study
Figure S1. Flow chart to present the experimental design of the present study. Figure S2. Volcano plot showing the differentially expressed genes between papillary renal cell carcinoma and non-cancer adjacent samples. Figure S3. The 2D molecular structures of the top 10 drugs. Table SI. The 60 potential drugs for papillary renal cell carcinoma treatment based on differently expressed genes. Drug name Dose Cell line Score Instance ID Pinacidil (C13H19N5) 16 µM HL60 -1 2406 Ciclosporin (C62H111N11O12) 3 µM HL60 -0.994 1331 Naftifine (C21H21N) 12 µM HL60 -0.966 2974 Vorinostat (C14H20N2O3) 10 µM HL60 -0.961 1161 Metacycline (C22H22N2O8) 8 µM HL60 -0.943 2901 Sulfacetamide (C8H10N2O3S) 16 µM HL60 -0.939 1859 Chlorprothixene (C18H18ClNS) 11 µM HL60 -0.938 1272 Amiodarone (C25H29I2NO3) 6 µM HL60 -0.933 2434 Noretynodrel (C20H26O2) 13 µM HL60 -0.914 1860 Valproic acid (C8H16O2) 200 µM HL60 -0.91 1155 Oxolinic acid (C13H11NO5) 15 µM HL60 -0.903 1419 Monocrotaline (C16H23NO6) 12 µM PC3 -0.881 7127 LY-294002 (C19H17NO3) 10 µM HL60 -0.869 1168 Aceclofenac (C16H13Cl2NO4) 11 µM PC3 -0.863 7269 Hyoscyamine (C17H23NO3) 14 µM HL60 -0.859 1424 Heliotrine (C16H27NO5) 13 µM HL60 -0.85 2180 Trichostatin A (C17H22N2O3) 1 µM HL60 -0.85 1175 Sulfachlorpyridazine (C10H8ClN4O2S) 14 µM HL60 -0.85 2191 Diflorasone (C22H28F2O5) 8 µM HL60 -0.838 2142 Yohimbine (C21H26N2O3) 10 µM MCF7 -0.833 6777 Sulfadiazine (C10H10N4O2S) 16 µM HL60 -0.833 1852 Pargyline (C11H13N) 20 µM PC3 -0.832 2102 Haloperidol (C21H23ClFNO2) 10 µM HL60 -0.829 6203 Methazolamide (C5H8N4O3S2) 17 -

Federal Register / Vol. 60, No. 80 / Wednesday, April 26, 1995 / Notices DIX to the HTSUS—Continued
20558 Federal Register / Vol. 60, No. 80 / Wednesday, April 26, 1995 / Notices DEPARMENT OF THE TREASURY Services, U.S. Customs Service, 1301 TABLE 1.ÐPHARMACEUTICAL APPEN- Constitution Avenue NW, Washington, DIX TO THE HTSUSÐContinued Customs Service D.C. 20229 at (202) 927±1060. CAS No. Pharmaceutical [T.D. 95±33] Dated: April 14, 1995. 52±78±8 ..................... NORETHANDROLONE. A. W. Tennant, 52±86±8 ..................... HALOPERIDOL. Pharmaceutical Tables 1 and 3 of the Director, Office of Laboratories and Scientific 52±88±0 ..................... ATROPINE METHONITRATE. HTSUS 52±90±4 ..................... CYSTEINE. Services. 53±03±2 ..................... PREDNISONE. 53±06±5 ..................... CORTISONE. AGENCY: Customs Service, Department TABLE 1.ÐPHARMACEUTICAL 53±10±1 ..................... HYDROXYDIONE SODIUM SUCCI- of the Treasury. NATE. APPENDIX TO THE HTSUS 53±16±7 ..................... ESTRONE. ACTION: Listing of the products found in 53±18±9 ..................... BIETASERPINE. Table 1 and Table 3 of the CAS No. Pharmaceutical 53±19±0 ..................... MITOTANE. 53±31±6 ..................... MEDIBAZINE. Pharmaceutical Appendix to the N/A ............................. ACTAGARDIN. 53±33±8 ..................... PARAMETHASONE. Harmonized Tariff Schedule of the N/A ............................. ARDACIN. 53±34±9 ..................... FLUPREDNISOLONE. N/A ............................. BICIROMAB. 53±39±4 ..................... OXANDROLONE. United States of America in Chemical N/A ............................. CELUCLORAL. 53±43±0 -

Evidence Review H: Management Options for Refractory Acne
National Institute for Health and Care Excellence Final Acne vulgaris: management [H] Management options for refractory acne NG198 Evidence reviews underpinning recommendations 1.5.15 and 1.5.16 as well as 1.6.1 to 1.6.4 in the NICE guideline June 2021 Final These evidence reviews were developed by the National Guideline Alliance which is a part of the Royal College of Obstetricians and Gynaecologists FINAL Error! No text of specified style in document. Disclaimer The recommendations in this guideline represent the view of NICE, arrived at after careful consideration of the evidence available. When exercising their judgement, professionals are expected to take this guideline fully into account, alongside the individual needs, preferences and values of their patients or service users. The recommendations in this guideline are not mandatory and the guideline does not override the responsibility of healthcare professionals to make decisions appropriate to the circumstances of the individual patient, in consultation with the patient and/or their carer or guardian. Local commissioners and/or providers have a responsibility to enable the guideline to be applied when individual health professionals and their patients or service users wish to use it. They should do so in the context of local and national priorities for funding and developing services, and in light of their duties to have due regard to the need to eliminate unlawful discrimination, to advance equality of opportunity and to reduce health inequalities. Nothing in this guideline should be interpreted in a way that would be inconsistent with compliance with those duties. NICE guidelines cover health and care in England. -

The Progestin Norethindrone Affects Sex Differentiation and Alters Transcriptional Profiles of Genes Along the Hypothalamic-Pitu
Aquatic Toxicology 201 (2018) 31–39 Contents lists available at ScienceDirect Aquatic Toxicology journal homepage: www.elsevier.com/locate/aqtox The progestin norethindrone affects sex differentiation and alters T transcriptional profiles of genes along the hypothalamic–pituitary–gonadal and hypothalamic–pituitary–adrenal axes in juvenile zebrafish Dario renio ⁎ Li-ping Houa, Hongxing Chenb, Chang-en Tiana, , Wen-Jun Shib, Ye Lianga, Rong-rong Wua, ⁎ Xu-wen Fanga, Cui-ping Zhanga, Yan-qiu Liangc, Lingtian Xieb, a School of Life Sciences, Guangzhou University, Guangzhou, 510006, China b The Environmental Research Institute, MOE Key Laboratory of Theoretical Chemistry of Environment, South China Normal University, Guangzhou, 510006, China c Faculty of Chemistry and Environmental Science, Guangdong Ocean Universtiy, Zhangjiang, 524088, China ARTICLE INFO ABSTRACT Keywords: Natural and synthetic progestins may pose a threat to wild fish populations living in receiving waters. In this Norethindrone study, the effects of norethindrone (NET) on the sex differentiation of zebrafish (Dario renio) and the mechanisms Sex differentiation Dario renio underlying these effects were investigated. Juvenile zebrafish (20 days post fertilization, pdf) were exposed to − Transcriptional expression environmentally relevant concentrations (5, 50, 500, and 1000 ng L 1) for 45 d. Sex ratio of the NET-exposed HPA and HPG populations, the histology of the gonads and the transcriptional profile of the regulatory genes involved in sex differentiation and steroidogenesis were examined. The results showed that a significantly higher ratio of male/ − female was induced in the zebrafish populations exposed to NET at concentrations higher than 32.3 ng L 1. Exposure to NET caused acceleration of sexual mature in males and a delay in ovary maturation in female zebrafish. -

Stembook 2018.Pdf
The use of stems in the selection of International Nonproprietary Names (INN) for pharmaceutical substances FORMER DOCUMENT NUMBER: WHO/PHARM S/NOM 15 WHO/EMP/RHT/TSN/2018.1 © World Health Organization 2018 Some rights reserved. This work is available under the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 IGO licence (CC BY-NC-SA 3.0 IGO; https://creativecommons.org/licenses/by-nc-sa/3.0/igo). Under the terms of this licence, you may copy, redistribute and adapt the work for non-commercial purposes, provided the work is appropriately cited, as indicated below. In any use of this work, there should be no suggestion that WHO endorses any specific organization, products or services. The use of the WHO logo is not permitted. If you adapt the work, then you must license your work under the same or equivalent Creative Commons licence. If you create a translation of this work, you should add the following disclaimer along with the suggested citation: “This translation was not created by the World Health Organization (WHO). WHO is not responsible for the content or accuracy of this translation. The original English edition shall be the binding and authentic edition”. Any mediation relating to disputes arising under the licence shall be conducted in accordance with the mediation rules of the World Intellectual Property Organization. Suggested citation. The use of stems in the selection of International Nonproprietary Names (INN) for pharmaceutical substances. Geneva: World Health Organization; 2018 (WHO/EMP/RHT/TSN/2018.1). Licence: CC BY-NC-SA 3.0 IGO. Cataloguing-in-Publication (CIP) data. -

Reproductive Health Guideline Appendix 3 – Search Strategies
SUPPLEMENTARY APPENDIX 3: Search Strategies 2020 American College of Rheumatology Guideline for the Management of Reproductive Health in Rheumatic and Musculoskeletal Diseases All searches initially run on 11/8/2017 and updated on 5/8/2018. Searches run from database inception to 5/8/2018. PUBMED Syntax Guide for PubMed [MeSH] = Medical Subject Heading [Text Word] = Includes all words and numbers in the title, abstract, other abstract, MeSH terms, MeSH Subheadings, Publication Types, Substance Names, Personal Name as Subject, Corporate Author, Secondary Source, Comment/Correction Notes, and Other Terms - typically non-MeSH subject terms (keywords)…assigned by an organization other than NLM [MeSH subheading] = a Medical Subject [Title/Abstract] = Includes words in the title Heading subheading, e.g. -

Noretynodrel (BAN, Rinn) 3
2120 Sex Hormones and their Modulators romethane. Protect from light. nortestosterone that have weak oestrogenic and andro- Activelle; Biofim†; Ciclovulon; Cliane; Estalis; Estalis SQ; Estracomb†; Estrag- USP 31 (Norethindrone Acetate). A white to creamy-white est; Gineane; Ginedisc 50 Plus†; Kliogest; Megestran†; Mericomb; Merigest; genic properties. They are commonly used as hormo- Mesigyna; Natifa Pro; Noregyna; Primosiston; Suprema; Systen Conti; Sys- odourless crystalline powder. Practically insoluble in water; sol- nal contraceptives (see p.2069). Norethisterone and ten Sequi; Trinovum†; Trisequens; Canad.: Brevicon; Estalis; Estalis Sequi; uble 1 in 10 of alcohol, 1 in less than 1 of chloroform, 1 in 2 of Estracomb; FemHRT; Loestrin 1.5/30; Minestrin; Ortho 0.5/35; Ortho 1/35; dioxan, and 1 in 18 of ether. norethisterone acetate are both given orally. Typical Ortho 7/7/7; Ortho-Novum 1/50†; Select 1/35; Synphasic; Chile: Activelle; daily doses are 350 micrograms for norethisterone and Cliane; Enadiol Neta; Estracomb; Estragest; Ginefolin; Kliogest; Mesigyna; Primosiston; Trisequens; Cz.: Activelle; Estalis; Estalis Sequi; Estrace Plus†; Norethisterone Enantate (BANM, pINNM) 600 micrograms for norethisterone acetate when used Estrace-C†; Estracomb†; Estragest†; Kliane; Kliogest; Menophase†; Non- alone, or 0.5 to 1 mg for norethisterone and 1 to 1.5 mg Ovlon†; Novofem; Pausogest; Sequidot; Systen Conti; Systen Sequi; Triak- Enantato de noretisterona; Norestisteron Enantat; Norethin- lim†; Trinovum; Trisequens; Denm.: Activelle; Econ†; Estracomb; Evo-Con- drone Enanthate; Noréthistérone, Enantate de; Norethisterone for norethisterone acetate when used with an oestro- ti; Evo-Sequi; Femanor; Femasekvens; Kliogest; Novofem; Ostranorm†; Tri- Enanthate; Norethisterone Heptanoate; Norethisteroni Enantas. gen. Norethisterone enantate is given by intramuscular norm†; Trinovum; Trisekvens; Fin.: Activelle; Estalis; Estalis Sekvens; Estracomb†; Evorel Conti; Evorel Sequi; Kliogest; Mericomb; Merigest; 17β-Hydroxy-19-nor-17α-pregn-4-en-20-yn-3-one heptanoate. -

Lactation and Reproduction *
HUMAN LACTATION Lactation and reproduction * A. M. THOMSON,1 F. E. HYTTEN,2 & A. E. BLACK 3 The authors review the literature on the effect of lactation on fertility in the absence of contraception and on the effects of contraceptive measures on lactation. They examine data from several countries on the intervals between births and on the return of menstru- ation and ovulation after childbirth, comparing lactating with nonlactating women. They conclude that lactation is an inefficient contraceptive for the individual, but that in popu- lations sustained lactation is associated with reduced fertility. Possible physiological mechanisms causing lactation amenorrhoea are discussed. Though much of the literature on the effect of contraceptives on lactation is inadequate, there is general agreement that the estrogen component of hormonal preparations has an adverse effect on lactation, but that progestins alone do not. Many questions remain. Is this effect seen in established lactation, or only in the puerperal period? Is it a direct pharmacological effect, or are pill-users the mothers least motivated to maintain breast-feeding? Does a close relationship exist between hormones given and lactation performance? The authors comment on some ofthe technical deficiencies ofprevious studies in thisfield and discuss practicalpossibilities of, and limitations to, obtaining adequate scientific information in the future. THE EFFECT OF LACTATION ON FERTILITY, enhanced and their sexual role as a female is height- IN THE ABSENCE OF CONTRACEPTION ened. We have far to go before we understand the function of post-partum taboos...". It is well established that a woman who is lactat- Return of fertility after a pregnancy can be gauged ing is less likely to become pregnant than one who in various ways, with varying degrees of certainty.